A Case of Effusive Myocarditis as a Result of the Immune Checkpoint Inhibitor, Nivolumab
Koshy, Gershon D.O.,
Hale, Andrew, D.O.,
Jaiswal, Dev D.O.,
Wildes, Daniel, D.O.,
Som, Mousumi, D.O.,
Abstract
Immune checkpoint inhibitors (ICIs) are approved for the treatment of many different cancers. Since their arrival, these agents have offered improved disease outcomes including a longer duration of overall survival. [1] There does remain however several uncommon yet severe adverse side effects from this drug class including cardiotoxicity.
Introduction
We observed a case of effusive myocarditis with pericardial effusion leading to cardiac tamponade as a result of the immune checkpoint inhibitor, nivolumab.
History of presentation:
A 71-year-old female with stage IV anorectal squamous cell carcinoma with metastases to her liver and paroxysmal atrial fibrillation was admitted to an oncologic specialty hospital for weakness, dysphagia, diplopia, and bilateral ptosis fourteen days after her initial nivolumab infusion. Her initial diagnosis was myasthenia gravis due to nivolumab. She was treated with pyridostigmine, intravenous immunoglobulin therapy, and high dose intravenous corticosteroids with improvement in symptomatology. On the third day of hospitalization, the patient developed acute onset of left-sided, sharp chest pain with associated shortness of breath. Electrocardiogram (ECG) demonstrated atrial fibrillation with a new left bundle branch block (LBBB) (figure 1).
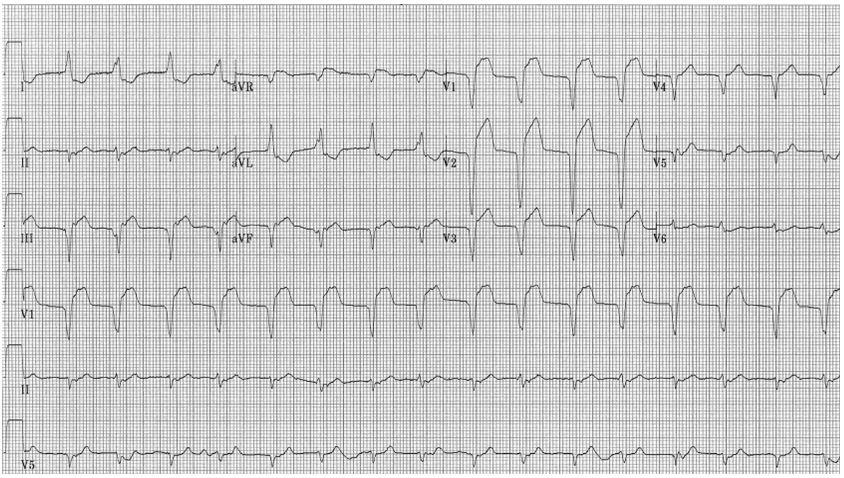
(Figure 1) Electrocardiogram demonstrating atrial fibrillation, left axis deviation, left bundle branch block.
Troponin I was found to be 1.3 (reference level, <0.05 ng/mL) and brain natriuretic peptide (BNP) was elevated at 880 (reference level, <100 pg/mL). Chest radiography revealed small bilateral pleural effusions and bibasilar opacities; otherwise, normal heart size and unremarkable pulmonary vasculature (figure 2). In the setting of chest pain, new LBBB, and elevated troponin, the patient was transferred to our acute tertiary care facility for emergent left heart catheterization

(Figure 2)Chest X-ray showing bibasilar opacities with obscuration of the bilateral costophrenic angles. Endotracheal tube terminating 3-4 cm above the carina, subcutaneous central venous port, and calcified bilateral breast implants.
The patient arrived at our facility in mild distress but was able to offer a brief description of her symptoms which included shortness of breath, orthopnea, and sharp chest pain prior to transport to the catheterization suite. Immediately before the procedure, the patient became increasingly somnolent and difficult to arouse. Due to concerns of her inability to protect her airway, the patient was intubated pre-procedurally. Left heart catheterization was then performed which revealed normal coronary arteries and left ventricular end diastolic volume (LVEDP) of 23 mmHg. During cardiac catheterization, the patient developed hypotension and electrocardiography displayed electrical alternans. Intraoperative echocardiogram was performed which revealed a moderate to severe pericardial effusion with collapse of the right atrium. Due to echocardiographic findings consistent with tamponade physiology, emergent pericardiocentesis was performed with placement of pericardial drain and immediate removal of approximately 250 ml of serous fluid leading to normalization of hemodynamics. A repeat, postcatheterization echocardiogram was performed which displayed a left ventricular ejection fraction of 40%, severe anterior and apical hypokinesis, and mild pericardial effusion without evidence of tamponade physiology. Of note, the patient's baseline echocardiogram performed 3 months prior to presentation showed an EF of 70%, with no gross wall motion abnormalities, and no significant valvular lesions.
The patient's case was discussed with her primary oncologist who recommended initiation of high dose IV corticosteroids (IV 1000 mg methylprednisolone) for myocarditis believed to be a
manifestation of her recent nivolumab therapy. The pericardial drain was later removed 48 hours post-pericardiocentesis after minimal output was noted from the drain and repeat transthoracic echocardiogram confirmed resolution of the pericardial effusion. Despite echocardiographic improvement, cardiac enzymes continued to rise during the patient's hospitalization and Troponin I ultimately peaked at a value of 23.7 ng/mL. Repeat ECG showed atrial fibrillation with prominent septal ST-segment elevation (figure 3); the ST segment elevation later included leads V4-V6 as well.
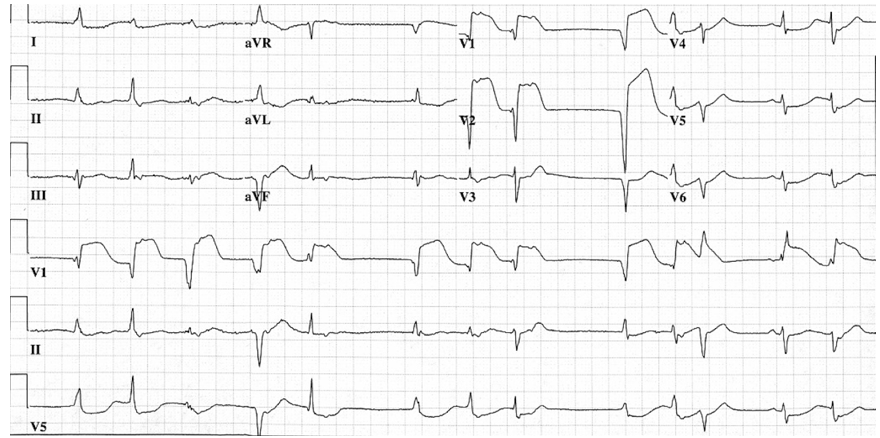
(Figure 3) Electrocardiogram demonstrating atrial fibrillation, left bundle branch block, and ST-elevation in septal leads V1-2 with lateral depression in leads V4-6, I, and aVL.
On day three of hospitalization, the patient was extubated to BIPAP and was able to speak with family and physician staff. Later that evening, she developed ventricular tachycardia with hemodynamic instability and was successfully cardioverted to normal sinus rhythm. Shortly after, the patient again became unstable and developed PEA (pulseless electrical activity) leading to advanced cardiac life support and reintubation. Return of spontaneous circulation achieved after 5-7 minutes of cardiopulmonary resuscitation.
Despite resuscitative efforts, the patient's condition remained guarded. Due to a lack of significant interval neurologic and cardiopulmonary improvement, a family discussion held several days later regarding goals of care led to the decision to transition the patient to comfort measures and aggressive treatment was discontinued at that time. Patient ultimately expired on day eight of hospitalization.
Discussion:
The ICI (immune checkpoint inhibitor), nivolumab, is a monoclonal antibody that blocks the PD-1 (programmed death-1) receptor which leads to dis-inhibition of tumor specific immune responses. [2] It has proved efficacious in the management of cancers involving nine organ systems including head and neck, skin, lymph node, breast, lung, liver, kidney, bladder, and ovarian.
Although this drug class has revolutionized the treatment of many malignancies and is for the most part considered well-tolerated, adverse reactions have been reported including myocarditis, dermatitis, endocrinopathies, colitis, hepatitis, and pneumonitis. [1] The mechanism for these inflammatory reactions are believed to be mediated through irregular activation of autoreactive T-cells.
Similar to our patient, there have also been other reported instances of myasthenia gravis following ICI therapy; some cases even with a concomitant myocarditis. [3] The immunohistological feature of this process involves macrophages resembling granulomas.
The cardiotoxicity associated with ICI-therapy is reported to be sudden in onset and quite severe relative to the other possible adverse side effects. As a result, recommendations have been made regarding the monitoring and management of ICI-associated cardiotoxicity. [4, 5] Patients that present with new cardiovascular symptoms or dysrhythmia identified on ECG while undergoing or following the completion of ICI therapy should undergo initial cardiovascular work-up, which includes additional ECG studies, cardiac enzymes, BNP or NT-pro-BNP, C-reactive protein, viral titer, and transthoracic echocardiogram. Endomyocardial biopsy should be considered if clinical suspicion is high despite unremarkable initial evaluation. If ICI-associated myocarditis is confirmed or suspicion otherwise remains high, ICI therapy should be withheld and administration of high dose corticosteroids (methylprednisolone 1000 mg/day on day 1, then oral prednisone 1mg/kg/day thereafter) should be started soon. Corticosteroids should only be discontinued once stabilization of cardiac enzymes, left ventricular ejection fraction, and electrocardiographic findings are achieved. Consideration for administration of immunosuppressive agents such as anti-thymocyte globulin, infliximab (unless decompensated heart failure is present), mycophenolate mofetil or abatacept should be given if corticosteroids offer no improvement or if the myocarditis is associated with hemodynamic instability. Resuming ICI therapy after an acute cardiotoxic event is generally discouraged; however, in the absence of alternative pharmacotherapies, consideration to resume ICI therapy is reasonable after discussion with the patient and a multidisciplinary team assessing risks and benefits.
Although endomyocardial biopsy and immunohistological evaluation was unable to be performed on our patient, the timeline of symptomatology and clinical course in relation to nivolumab therapy in the setting of normal baseline cardiac function is suggestive of an ICI mediated cardiotoxic event. With the exception of hypertension, the patient had no other cardiovascular disease risk factors and normal cardiac function was demonstrated by the unremarkable baseline echocardiogram prior to initiation of immunotherapy. The patient's new onset cardiomyopathy was then confirmed to be non-ischemic after no significant coronary disease was identified during left heart catheterization.
The Naranjo ADR Probability Scale is a 10 question scoring tool used to assess and stratify the likelihood that a medication in question directly caused an adverse event. [6] When applied to
our patient's case, the ADR Probability Scale indicated a possible relationship between the nivolumab and the effusive myocarditis. There are however limitations in the application of this tool as certain components of the scoring device including dosage changes, lack of previous administration, lack of placebo, and inability to detect drug concentrations were either not applicable or unable to be assessed.
Though immune check point inhibitors have overall improved outcomes of several oncologic disorders, cardiac structure and function should be closely evaluated prior to initiation and subsequent interval monitoring must be performed with the aim of preventing severe, cardiotoxic outcomes.
References:
1. Wolchok JD. PD-1 blockers. Cell. 2015;162(5):937.
2. Johnson DB. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. New England Journal of Medicine. 2016;375(18):1749-1755. doi:10.1056/nejmoa1609214.
3. Rota E. Concomitant myasthenia gravis, myositis, myocarditis and polyneuropathy, induced by immune-checkpoint inhibitors: A life-threatening continuum of neuromuscular and cardiac toxicity. Clinical Neurophysiology. 2019;130(1). doi:10.1016/j.clinph.2018.09.071.
4. Curigliano G. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Annals of Oncology. 2020;31(2):171-190. doi:10.1016/j.annonc.2019.10.023.
5. Palaskas N. Immune Checkpoint Inhibitor Myocarditis: Pathophysiological Characteristics, Diagnosis, and Treatment. Journal of the American Heart Association. 2020;9(2). doi:10.1161/jaha.119.013757.
6. Naranjo CA. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-45.


