How to Differentiate Between Varying Types of Diabetes in Adult Patients
Lauren Calnan, PharmD, BCCCP, Hillcrest Hospital South, Tulsa, OK
Laura Holliday, PharmD
Oklahoma State University Medical Center, PGY2 Pharmacy Resident, Tulsa, OK
Kailyn Ogle, PharmD, Oklahoma Heart Hospital South, Oklahoma City, OK
Jeremy L. Johnson, PharmD, BCACP, CDCES (CDE), BC-ADM, Associate Professor, Southwestern Oklahoma State University College of Pharmacy, Department of Pharmacy Practice, Weatherford, OK;
Clinical Pharmacist, Ambulatory Care, Diabetes Care & Education Specialist, OSU Medicine, Department of Internal Medicine, Tulsa, OK.
Key words: latent autoimmune diabetes of adults, LADA, types of diabetes, type 1 diabetes, type 2 diabetes
Abstract
Diabetes is a condition that clinicians are faced with treating in a wide variety of practice settings. The great majority of cases are diagnosed as either type 1 diabetes (T1DM) or type 2 diabetes (T2DM). Latent autoimmune diabetes in adults (LADA) is a form of diabetes comparable to T1DM in that it results from autoimmune destruction of pancreatic beta cells and requires treatment with insulin therapy. In contrast to T1DM, LADA presents much later in life and, thus may be diagnosed as T2DM. In some cases, none of these three types can be definitively diagnosed, implying there must be other types to identify. The case presented illustrates this; a patient diagnosed with T2DM was later suspected to be have LADA or an undetermined type of diabetes. Initially thought to be rare, LADA is being identified more frequently. By bringing awareness to the disease, more patients will be aptly diagnosed and receive the appropriate treatment sooner in the course of their disease.
Introduction
Diabetes is an epidemic with catastrophic consequences if left untreated or misdiagnosed. In the United States alone it is the seventh leading cause of death, and it is estimated more than thirty million people are living with this diagnosis.1 The most common diagnoses for diabetes include T1DM, T2DM, and gestational diabetes. However, there is an estimated 10% of all diabetes cases that may be more appropriately diagnosed as latent autoimmune diabetes in adults (LADA).2-4 LADA is a slowly progressing form of autoimmune diabetes, compared to T1DM, and is defined by adult age at onset, an initially-delayed need for insulin therapy, and the presence of diabetes-associated antibodies.2, 5-8 Its mixed presentation often leads to initial misdiagnosis as T2DM due to adult onset and a limited initial response to lifestyle improvements and some oral medications. Incorrect management of LADA may lead to poor glycemic control and a delay in the proper pharmacologic treatment may increase risk of complications.
T1DM is characterized by the autoimmune destruction of pancreatic beta cells. This destruction of beta cells is the result of the inappropriate generation of diabetes associated-autoantibodies such as islet-cell antibodies (ICAs), glutamic acid decarboxylase (GAD) antibodies, protein tyrosine phosphatase IA-2 (IA-2) antibodies, or insulin autoantibodies (IAA).2, 4 As a result, patients with T1DM retain little to no insulin production, and therefore, require insulin injections for survival. T1DM patients often encounter a trigger (such as certain viruses) and are typically diagnosed at an early age, present underweight due to weight loss from the body's inability to utilize glucose as an energy source and subsequent breakdown of fatty stores, and have the potential to transition into ketosis resulting in diabetic ketoacidosis (DKA).9 In contrast, T2DM results from genetic and environmental factors. Over time, these factors lead to development of insulin resistance and insufficient insulin production, among other mechanisms that lead to hyperglycemia.4, 5 While these patients are still able to produce insulin, when wildly uncontrolled, T2DM patients may go into a hyperosmolar hyperglycemic state (HHS) or DKA. While DKA is possible in these patients, it is not expected. However, T1DM patients are predisposed to DKA.9 Patients diagnosed with T2DM are usually adults at onset and are often overweight to obese (Body Mass Index (BMI) >25 to >30 kg/m²). The treatment options range from lifestyle modifications to oral anti-diabetic agents to insulin therapy.
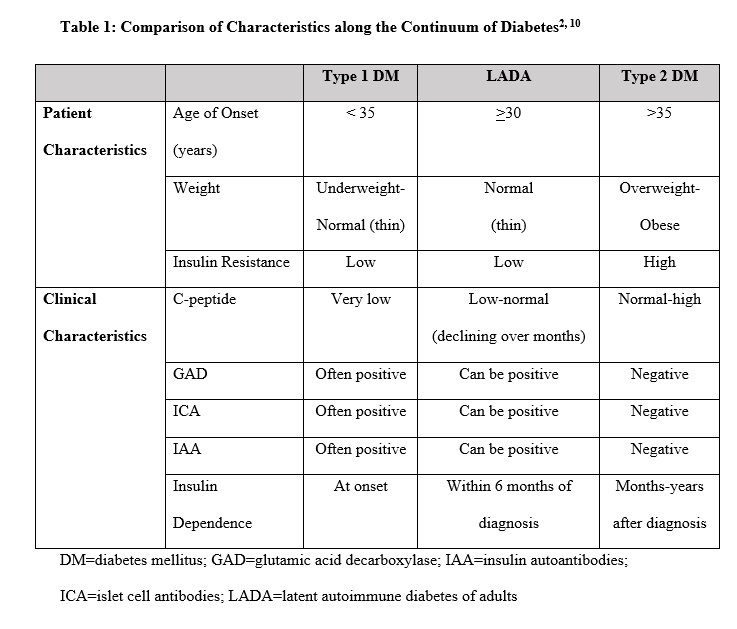
Lab values such as A1C, fasting plasma glucose, random plasma glucose, or oral glucose tolerance test readings above normal thresholds are used for initial diagnosis of diabetes, but these are not indicative of one particular type. Often, the specific diagnosis of either T1DM or T2DM seems fairly intuitive based on patient and clinical characteristics (Table 1). Patients with T1DM are often diagnosed during childhood, appear thin with a BMI underweight to normal (<18 to 24 kg/m2), and rapidly require insulin after diagnosis. Many individuals often experience DKA with extremely elevated blood glucose levels, rapidly lose control with illness/infection, and are sensitive to even small insulin adjustments. Conversely, patients with T2DM are often diagnosed during adulthood, appear overweight to obese with a BMI >25 to >30 kg/m2, and initially may respond well to lifestyle modifications and/or non-insulin glucose-lowering agents. They rarely experience DKA when gross glucose elevations occur, but may experience HHS, and often are resistant to insulin, requiring larger doses to gain glucose control once insulin is eventually needed. These patterns fit the pathophysiology of each type of diabetes. However, not all patients present with such a clear set of characteristics. What does it mean when a patient has a mixed picture of characteristics and lab findings? Such a patient could represent LADA or potentially a newly recognized type or subgroup of diabetes; an example of how this scenario was approached is presented in the following case.
Patient Case
A 66 year-old African American male, regularly seen by internal medicine residents in an academic outpatient clinic, had been diagnosed with T2DM for 10 years. His past medical history was also significant for treated hepatitis C with cirrhosis, hyperlipidemia, hypertension, stroke, and adrenal nodule. During his care, he had been prescribed metformin 500 mg daily which was titrated up to 1000 mg twice daily, sitagliptin 100 mg daily, and various titrated doses of insulin detemir and insulin aspart. His A1C had varied widely over the previous 2 years from 5.8% (40 mmol/mol) to 11.0% (97 mmol/mol). After being hospitalized with DKA and with an increasing A1C, he was referred to the clinic's ambulatory care clinical pharmacist for evaluation and further education and management of his diabetes.
At his initial visit with the clinical pharmacist, he reported he had been adherent with his oral medications, metformin 1000 mg twice daily and sitagliptin 100 mg daily, but was incorrectly administering his insulin regimen, resulting in under-utilization of insulin and complete lack of insulin use over the previous two weeks. His A1C was 11.5% (102 mmol/mol) and providers were skeptical of the accuracy of his reported Self Monitoring Blood Glucose (SMBG) levels and believed his insulin use to be very inconsistent and infrequent. His BMI was 21.59 kg/m2 and he appeared thin. He was sensitive to small insulin dose adjustments, had a history of DKA, and rapidly lost control of his glucose with illness, much like someone with T1DM. The clinician provided education to the patient for appropriate timing and administration of insulin and SMBG checks relative to meals for a fixed-dose pattern-management approach to care, as well as discussion of LADA testing with potential for medication changes at the next visit.
Antibody testing was obtained and revealed IAA at 5.5 U/mL (normal <0.2 U/mL), C-peptide 3.3 ng/mL (normal 1.1-4.4 ng/mL), and GAD of <5 U/mL (normal <5 U/mL). At the patient's 1-week follow up appointment, based on clinical characteristics and the positive IAA, the clinicians discussed the potential diagnosis of LADA. However, a LADA diagnosis was still not definitive. When serum samples were tested for beta cell specific autoantibodies, only insulin autoantibodies were detected (IAA). The finding of IAA alone in a patient who has been treated with insulin for 10 years is not enough to define this patient as LADA since insulin antibodies may be found in subjects that have been using an insulin regimen prior to testing for IAA. The diagnosis would have been founded if GAD autoantibodies or ICAs had been detected. Additionally, this patient had normal C-peptide levels 10 years after diagnosis, which is more fitting with T2DM. Many of the patient and clinical characteristics fit into both T1DM and T2DM, leading to the suspicion of LADA. Due to the patient's inconsistent insulin adherence, it was decided to continue oral medications at that time and adjust his insulin regimen along with education of appropriate administration and reinforced SMBG tracking. The patient agreed to check his SMBG before meals and at bedtime. The possibility of being insulin dependent in the future was relayed, along with the likelihood of stopping his oral medications for diabetes.
After this education session, the patient began to administer insulin at appropriate times with meals and displayed adherence with both his insulin regimen and oral medications. Over the next few visits, SMBG levels greatly improved. At his last visit, his SMBG logs revealed the following average blood glucose ranges: breakfast pre-prandial 100-119 mg/dL (5.5-6.6 mmol/L), lunch pre-prandial 125-155 mg/dL (6.9-8.6 mmol/L), and dinner pre-prandial 140-165 mg/dL (7.8-9.2 mmol/L) (the patient never checked bedtime SMBG). The patient was ultimately using insulin detemir 11 units twice daily, insulin aspart 5 units with meals, metformin 1000 mg twice daily, and sitagliptin 100 mg daily. His A1C began to downtrend, with the most recent at 8.1% (65 mmol/mol), down from the 11.5% (102 mmol/mol) three months prior when he experienced DKA. Clinicians believed that with more time and fine-tuning of his insulin regimen, his A1C would have continued to improve to goal of <7% (53 mmol/mol). However, when the patient did not arrive for his next appointment, it was determined that he had been admitted to the hospital for complications due to hepatocellular carcinoma and, unfortunately, expired.
Discussion
This patient does not fit the perfect diagnostic molds for either T1DM or T2DM, and if he does not perfectly fit the criteria for LADA either, then perhaps this identifies a need for other diagnostic options. If this case does not represent a patient with LADA, it does demonstrate that patients can present with very mixed pictures, which muddy the diagnostic waters. Regardless, a more appropriate treatment approach was identified for this patient upon recognizing his mixed characteristics. This case also illustrates the heterogeneous nature of LADA and the need to define other potential types of diabetes. Providing an accurate diagnosis is crucial to helping a patient obtain glycemic control, prevent complications, and avoid unnecessary frustration.
Novel methods to differentiate between types of diabetes is imperative. Al-Majdoub et al attempted to identify a metabolic profile for LADA distinguishable from both T1DM and T2DM.11 It was found that LADA characteristics collectively had metabolic markers indicative of both T1DM and T2DM, however, C-peptide level was most heavily correlated with each respective condition. This could be helpful in diagnosing LADA more efficiently by serving as a differential marker between it and T2DM. The authors suggest that many patient and clinical characteristics overlap among these three types of diabetes, which all seem to fall on a continuum, and that LADA patients fall intermediate to either T1DM or T2DM with characteristics more closely resembling T2DM. They also imply that LADA patients more closely resembling T2DM may have a slower progression to insulin therapy than those more resembling T1DM; further exposing the complexities of discriminating between these three types of diabetes. Castelblanco et al orchestrated a similar study comparing inflammatory marker profiles between LADA, T1DM, and T2DM.12 Soluble tumor necrosis factor-receptor 2 (sTNFRII) and high-sensitivity C-reactive protein (hs-CRP) were found to be helpful in differentiating between T1DM and LADA.12 Perhaps these markers can be of future use in specifying a proper diagnosis. Though they can be costly and burdensome, completing a thorough assessment of laboratory values may prove to be worthwhile if it means achieving an accurate diagnosis.
This appreciation of variations of diabetes on a spectrum implies that there could be many different types of diabetes to appreciate and best manage in varying approaches on a continuum of insulin requirements. If T1DM falls at one extreme end of the spectrum which requires insulin therapy early, and T2DM falls at the opposite end allowing non-insulin therapies early, then recognizing certain characteristics may help clinicians to better predict the most appropriate course of early treatment after diagnosis. By our current definitions, some LADA patients may require insulin earlier than others, but perhaps these patients should be assigned a new name for their type of diabetes. Supporting this theory, Ahlqvist et al recently identified five subgroups of adult-onset diabetes based on clusters of traits possessed by patients at diagnosis.13 T1DM and LADA are grouped together while the other four seem to be subsets of what we currently appreciate to be T2DM. Features of each cluster are as follows: Cluster 1 (Severe autoimmune diabetes): early disease onset, relatively low BMI, poor metabolic control, insulin deficiency (impaired insulin production), and GAD antibody +; Cluster 2 (Severe insulin-deficient diabetes): similar to cluster 1 but GAD antibody -, high A1c, and high incidence of retinopathy; Cluster 3 (Severe-insulin resistant diabetes): insulin resistance, high BMI, and highest incidence of nephropathy; Cluster 4 (Mild obesity-related diabetes): obesity, younger age, and not insulin resistant; and Cluster 5 (Mild age-related diabetes): older age and modest metabolic alterations.14 The authors point out that identifying a specific cluster at diagnosis could help clinicians better customize initial treatment approaches or therapies to gain glucose control and slow progression of disease.13 In contrast to Ahlqvist et al, Dennis et al found that among those with T2DM, using simple clinical features (age at diagnosis for glycemic progression and baseline renal function for renal progression) for predicting patient response to therapy and potential outcomes outperformed the cluster approach regarding prediction of glucose-lowering response to select therapy for individual patients. The results suggest modelling clinical features directly may provide greater clinical utility than using clinical features to place patients into subgroups .15 There are no formal standardized diagnostic criteria for LADA, which creates a challenge when trying to define proper treatment for the condition. Proposed diagnostic criteria from the Immunology of Diabetes Society (IDS) suggests that defining criteria for LADA include a positive test for at least one antibody associated with T1DM, a minimum age of 30 years at onset, and the patient was not treated with insulin within the first 6 months of diagnosis (the delayed insulin use criterion differentiates LADA from T1DM).2, 10 It is still up for debate whether or not patients who are originally diagnosed with T2DM should be tested for antibodies upon diagnosis. Some studies support this action, arguing that LADA may be more prevalent than originally thought.16
Because it is a subtype of T1DM, LADA treatment will eventually emulate that of T1DM requiring insulin as the base of therapy. There is no established intervention strategy for the condition, however, therapies that can preserve beta cell function may, theoretically, have a role in slowing the progression to insulin dependence. Evidence could support use of glucagon-like peptide 1 receptor agonists, dipeptidyl peptidase-4 inhibitors, thiazolidinediones, or even interleukin-1 receptor antagonists or a GAD vaccine (antigen-specific immunomodulation).2, 10, 17-19 Sulfonylureas have been shown to decrease the time to insulin dependence, result in poorer glycemic control compared to treatment with insulin16, and should likely be avoided. Use of appropriate therapeutic agents to slow progression would require early recognition of patient and clinical characteristics to properly identify LADA, both for the purpose of initiating therapy that preserves beta cell function and recognizing the proper time to move to insulin therapy. A patient misdiagnosed with T2DM and initiated on typical non-insulin treatments would, over months, begin to lose response to these medications. The resultant rise in SMBG levels could be misinterpreted as non-adherence with diet and/or medications; thus, causing the clinician to titrate to higher doses of the ineffective medications and further counsel on lifestyle interventions to no avail, resulting in a potentially long delay in the proper switch to basal/bolus insulin therapy.
Conclusion
Clinicians are recognizing LADA more regularly than in the past, however, many patients are still incorrectly diagnosed with T2DM based on adult age at diagnosis. The case presented in this report highlights the overlap of various characteristics among patients with different types of diabetes and the difficulties that can cloud diagnosis. LADA may be even more prevalent than T1DM once all cases have been accounted for. The abundance of diagnoses of T2DM may someday be divided into better-understood and more-appropriate types or subgroups of diabetes. Appreciation of characteristics that are more associated with LADA should lead to many more, proper diagnoses, and therefore, appropriate management of diabetes. Otherwise, a delay in optimal treatment could produce more-rapid development of diabetic complications. More research is needed for continued identification of pathophysiologic mechanisms of LADA on the continuum of diabetes and identification of new types of diabetes. Efforts to better educate clinicians on these infrequently encountered disease types are important to improve recognition and proper treatment.
Author Contributions
LC and JLJ wrote the manuscript, reviewed/edited the manuscript, researched data, and contributed to the discussion. LH wrote the manuscript, reviewed/edited the manuscript, and contributed to the discussion. KO wrote the manuscript and reviewed/edited the manuscript. JLJ is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
1. Centers for Disease Control and Prevention. Diabetes, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017.
2. Naik R, Brooks-Worrell B, Palmer J. Latent autoimmune diabetes in adults. J Clin Endocrinol Metab. 2009;94(12):4635-4644.
3. Stenstrom G, Gottsater A, Bakhtadze E, et al. Latent autoimmune diabetes in adults: definition, prevalence, beta-cell function, and treatment. Diabetes. 2005;54:S68-72.
4. Nambam B, Aggarwal S, Jain A. Latent autoimmune diabetes in adults: a distinct but heterogeneous clinical entity. World Journal of Diabetes. 2010;1(4):111-15.
5. Pozzilli P, Di Mario U. Autoimmune diabetes not requiring insulin at diagnosis (latent autoimmune diabetes of the adult). Diabetes Care. 2001;24:1460-67.
6. Redondo, M. LADA: time for a new definition. Diabetes. 2013;62(2):339-40.
7. Leslie RD, Williams R, Pozzilli P. T1DM and latent autoimmune diabetes in adults: one end of the rainbow. J Clin Endocrinol Metab. 2006;91:1654-59.
8. Niegowska M, Delitala A, Pes GM, et al. Increased seroreactivity to proinsulin and homologous mycobacterial peptides in latent autoimmune diabetes in adults. PLoS ONE. 2017;12(5):e0176584.
9. Kitabchi AE, Umpierrez GE, Murphy MB, et al. Management of Hyperglycemic crises in patients with diabetes. Diabetes Care. 2001;24(1):131-53.
10. O'Neal KS, Johnson J, Panak RL. Recognizing and appropriately treating latent autoimmune diabetes in adults. Diabetes Spectrum. 2016;29(4):249-52.
11. Al-Majdoub M, Ali A, Storm P, et al. Metabolite profiling of LADA challenges the view of a metabolically distinct subtype. Diabetes. 2017;66(4):806-14.
12. Castelblanco E, Hernandez M, Castelblanco A, et al. Low-grade inflammatory marker profile may help to differentiate patients with LADA, classic adult-onset T1DM, and T2DM. Diabetes Care. 2018;41(4)862-68.
13. Ahlqvist E, Storm P, Karajamaki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes and Endocrinology. 2018;6(5):361-9. DOI: https://doi.org/10.1016/S2213-8587(18)30051-2.
14. Davenport L. Diabetes consists of five types, not two, say researchers. Medscape March 01, 2018. Accessed July 10, 2018, at https://www.medscape.com/viewarticle/893305?src=wnl_tp10hc_180628_mscpedit_pulm&uac=25797MN&impID=1671168&faf=1#vp_2.
15. Dennis JM, Shields BM, Henley WE, Jones AG, and Hattersley AT. Disease progression and treatment response in data-driven subgroups of T2DM compared with models based on simple clinical features: an analysis using clinical trial data. Lancet Diabetes Endocrinol. 2019; http://dx.doi.org/10.1016/S2213-8587(19)30087-7
16. Brophy S, Davies H, Mannan S, et al. Interventions for latent autoimmune diabetes (LADA) in adults. Cochrane Database Syst Rev. 2011;9.
17. Yang Z, Zhou Z, Li X, Huang G, Lin J. Rosiglitazone preserves islet β-cell function of adult-onset latent autoimmune diabetes in 3 years follow-up study. Diabetes Res Clin Pract. 2009;83:54-60.
18. Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1 receptor antagonist in T2DM. N Engl J Med. 2007;356:1517-1526.
19. Agardh CD, Cilio CM, Lethagen A, et al. Clinical evidence for the safety of GAD65 immunomodulation in adult-onset autoimmune diabetes. J Diabetes Complications. 2005;19:238-246.