Dysphagia Lusoria; Aberrant Right Subclavian Artery with Bicarotid Trunk as a Cause for Dysphagia
Marshall Harris D.O., Oklahoma State University Medical Center
Weston Zickgraf D.O., Oklahoma State University Medical Center
Christos Vassilou D.O., Oklahoma State University Medical Center
Donald von Borstel D.O. Oklahoma State University Medical Center
Funding associated with this manuscript: No funding was received to produce this manuscript.
Conflicts of Interest: No conflicts of interest to disclose with the production of this manuscript.
Key Words: Aberrant Right Subclavian Artery, Dysphagia Lusoria, Bicarotid Trunk, Dysphagia
Abstract
Dysphagia lusoria is the medical term used to describe the co-occurrence of dysphagia in the setting of a congenital vascular anomaly causing extrinsic compression upon the esophagus, most commonly an aberrant right subclavian artery. The majority of aberrant vasculature are incidental findings without associated symptoms, however those with symptoms may receive treatment in the form of conservative therapy and surgical correction if needed. We present a complex case of a patient with aberrant right subclavian artery and bicarotid trunk causing dysphagia lusoria and dyspnea. The patient was diagnosed by esophagram and computed tomography angiogram, failed conservative management, and ultimately required surgical correction. This case report focuses on the imaging and therapeutic options in these patients, as well as additional points to consider in the evaluation and management of patients with dysphagia lusoria.
Introduction
Aberrant right subclavian artery (aRSA) is the most common aortic arch anomaly, excluding the bovine aortic arch variant. It is seen in 0.5% to 2% of the general population, usually asymptomatic and noted as an incidental finding1. aRSA is a congenital variant that forms through regression of the right ductus arteriosus and the segment of the right aortic arch between the right common carotid and right subclavian artery. This results in a retropharyngeal course of the right subclavian artery as the vessel originates from the dorsal distal aortic arch, as opposed to the right fourth arch2. The majority of aRSA’s cross midline in the retro-esophageal space, some cross between the trachea and esophagus and still others cross anterior to both the trachea and esophagus with occurrence rates estimated at 80%, 15%, and 5% respectively1.
When an aRSA does cause symptoms, it most commonly presents as dysphagia, which was present in 91% of reported cases in one study1. Originally coined in 1794, London surgeon David Bayford called it “dysphagia lusus naturae”, now called dysphagia lusoria, which is Latin for ‘freak of nature’.3,4 Other less common presenting symptoms include chest pain, sensation of mechanical obstruction, postprandial bloating and regurgitation of unchewed foods. In infants, although uncommon, this may present as respiratory distress from extrinsic compression on the infantile trachea given its lack of rigidity1. aRSA typically causes no symptoms, especially in the young adult population as in our case report, and for this reason it would be abnormal for someone to present in such a way that it would limit their daily activities.
Case Report
A 34-year-old female presented via referral to a general surgery clinic with 2-3 years of worsening dysphagia, chest pain, dyspnea, and the sensation of “food getting stuck” in her throat. Her pertinent past medical history was significant for asthma and well controlled diabetes mellitus type II. She had a family of heart disease. The patient was an avid long-distance runner but had to decrease the length of her runs secondary to worsening chest pain and dyspnea. Her remaining social history was predominately non-contributory for the purposes of this case report.
Due to the patient’s dyspnea and chest pain limiting her ability to exercise, the patient underwent a screening electrocardiogram which was negative.
The initial presumptive diagnosis was gastroesophageal reflux disease managed with over-the-counter omeprazole 20mg daily, lifestyle modification, and dietary adjustments. She experienced mild relief of symptoms with this management, however often suffered from upper abdominal pain. These continued symptoms resulted in the patient getting a right upper quadrant ultrasound that displayed cholelithiasis. She underwent a successful cholecystectomy, which did not ultimately improve her symptoms.
As the patient’s symptoms worsened, a fluoroscopic upper gastrointestinal (GI) system double-contrasted air and barium study was performed to evaluate for potential esophageal disorders. The study demonstrated a left-sided posterior extrinsic compression and narrowing of the proximal-to-mid esophagus (Figures 1 & 2). Through this region there was delayed motility of the swallowed barium. Additionally, there was a mild to moderate amount of distal esophageal reflux of contrast. This compression was cranial to the expected slight normal aortic arch contour of the esophagus which prompted follow-up diagnostic evaluation with a computed tomography angiogram (CTA) of the chest to evaluate for potential variant vascular anatomy. CTA was performed 6 days after the upper GI which revealed an aRSA crossing midline in the retro-esophageal space (Figures 3 & 4). Additionally relevant was variant vascular anatomy of a common origin of the right and left common carotid arteries, stemming from a single bicarotid trunk. This vascular anomaly is termed a bicarotid trunk, or truncus bicaroticus (Figures 3 & 4).

Figure 1: Frontal fluoroscopic projection of the patient’s chest after ingestion of effervescent granules followed by liquid barium contrast revealing an extrinsic compression of the left proximal to mid-esophagus in the region of the great vessels (A.) with zoomed in projection of the abnormality (B.).

Figure 2: Oblique fluoroscopic projection upper GI study revealing diagonally oriented, extrinsic compression noted on the posterolateral aspect of the patient’s esophagus (thick white arrow). Note the amount of pooled contrast material proximal to the site of compression, consistent with delayed transit.
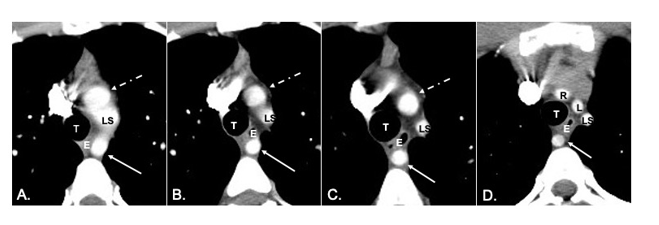
Figure 3: Sequential axial slices of CTA with IV contrast of the chest, labeled A.-D. These images are in order from caudal to cranial demonstrating a retro-esophageal course of an aRSA (solid thin arrow) as it courses towards the right thorax. Additionally, there is common origin of the right and left common carotid arteries, stemming from a bicarotid trunk (dotted thin white arrow) with bifurcation of right and left common carotid arteries in D. LS, left subclavian artery; T, trachea; E, esophagus; L, left common carotid artery; R, right common carotid artery;

Figure 4: Single sagittal CTA image (A.) with oblique CTA maximum intensity projection (MIP) images (B. and C.) of the patient’s chest demonstrating retro-esophageal course of a right aberrant subclavian artery (black arrow). Also of significance, is the common origin of the right and left common carotid arteries, stemming from a bicarotid trunk (white arrow) and best visualized on image C. T, trachea; LS, left subclavian artery; E, esophagus; r, right common carotid artery; L, left common carotid artery; AA, aortic arch; LB, left mainstem bronchus; DA, descending thoracic aorta;
Two months after the CTA was obtained, the consulted cardiovascular surgeon performed a successful right thoracotomy with ligation of the origin of the aRSA and ascending aortic-to-right subclavian artery bypass using a 6mm woven velour vascular graft. She had a relatively uneventful postoperative course and follow-up 4 weeks later showed improved dyspnea and dysphagia. However, 5 months after aRSA ligation the patient returned to her primary care provider with worsening dysphagia and reflux which was treated by increasing her omeprazole to 40mg daily, and ultimately lost to follow-up.
Discussion
Throughout the medical literature there is a widely shared view that in isolation, an aberrant subclavian artery usually will not cause symptoms in the adult population due to the distensibility of the esophagus and space for motion to occur when necessary5. Multiple mechanisms are offered as an explanation for why an aRSA can be symptomatic, including increased rigidity of the esophagus or the vessel wall (ie: atherosclerotic vascular disease) or aneurysmal dilation of the aortic arch or aberrant subclavian artery. Other mechanisms for symptomatic aRSA includes Kommerell’s diverticulum, a focal dilation of the origin of aberrant subclavian artery which is commonly seen, or elongation of the aorta that is commonly seen with aging. Lastly, the presence of a common origin bicarotid trunk in addition to an aberrant
subclavian artery can be another etiology for symptomatic aRSA5. If there is one or more of the above findings in association with an aberrant vessel, the patient is postulated to have a more favorable surgical outcome if non-surgical management fails. Those who do not share any of the above findings, should exhaust all non-surgical options before deciding surgical management is necessary. This is because there is controversy regarding whether dysphagia lusoria is even caused by aberrant vasculature, or if an incidental finding of aberrant vasculature is blamed for symptomatic gastroesophageal reflux disease or another common disorder causing symptoms.
Initial workup of dysphagia with concern for vascular compression starts with double-contrasted fluoroscopic upper GI study or esophagram which shows a characteristic diagonal impression on the posterior aspect of the esophagus. This is then followed by CT angiogram or digital subtraction angiography for the diagnosis of aberrant vasculature. It can also be seen as a pulsatile extrinsic mass by endoscopic visualization. Conservative management of dysphagia lusoria is aimed at acid reduction in the form of proton pump inhibitors, lifestyle modifications, and dietary changes such as limiting spicy or acidic foods. Prokinetic medications, namely cisapride has been used with reasonable outcomes with the intention of propelling swallowed bolus past the compression site, however, is infrequently used anymore. This is because the dysphagia may be a result of esophageal impression by vascular ring itself, or by motility changes5. When acute, dysphagia in adults can sometimes be treated with intravenous glucagon, which allows distal sphincter relaxation if a food bolus is stuck6. Nonacute treatment should be aimed toward the underlying cause or diagnosis and, if non-surgical therapy is warranted, speech pathologist consultation with speech therapy may be of clinical benefit. Obtaining a specific solid and/or liquid diet can provide relief whenever specific solids or liquids provoke symptoms done by speech pathologist or dietician, which would also include thickening or thinning substances as needed7. Swallowed bread bolus soaked in barium may be helpful in the evaluation of abnormal peristalsis and/or delayed transit of solid foods. In patients who are poor surgical candidates or in scenarios of temporizing symptoms, endoscopic dilation by gastroenterologist may be performed however should not be considered definitive management8.
Finally, if dysphagia and related symptoms are unrelenting to appropriate conservative therapy, surgical correction should be considered. Many different techniques have been described, including a supraclavicular approach, median sternotomy, and right or left lateral thoracotomy approaches which typically coincide with the type of aberrant artery. The typical procedure involves proximal ligation at the origin of the aberrant vessel, with graft placement either an end-to-side anastomosis of the carotid artery or by anastomosis to the ascending aorta itself8. Residual symptoms of dysphagia have been reported in cases of ligation where the proximal-most aspect of artery remains as a long stump, therefore some advocate for a left thoracotomy for adequate access and proper full ligation of the arterial origin on descending aorta9. There is risk of developing subclavian steal in the scenario of an end-to-side carotid anastomosis. Dysphagia lusoria caused by aneurysm of the aberrant artery or aneurysm of the aorta itself is managed by endovascular stenting. This is because of the higher risk of mortality associated with aneurysmal rupture in relation to mortality from surgical correction of the aberrant artery itself. Dysphagia lusoria caused by aneurysm is a major indication for surgical correction versus conservative management11.
Conclusion
Our case displays a unique presentation of a common anatomic variant. While the patient did not ultimately portray a perfect outcome of surgical management, there are multiple points to consider as to why this was the case, and why this case was of academic value. The patient had a previous diagnosis of asthma, and it is reasonable to consider infantile shortness of breath from tracheal compression from her vascular ring as a cofounding factor to her symptomatology as the infantile and pediatric trachea does not have the same rigidity as adults. As seen on her CTA, she had a bicarotid trunk in addition to the aRSA which has been associated with development of dysphagia lusoria as opposed to an aRSA in isolation. This has been proposed to be related to the limited anterior motion of the trachea and esophagus further exacerbating the limited motility of swallowed contents through this region10. Surgical correction did appear to have a positive impact on the patient’s dyspnea and initially improved her dysphagia. However, the return of symptoms 5 months later tells us this pathology is complicated in etiology and treatment.
References
1. Levitt B, Richter JE. Dysphagia lusoria: a comprehensive review. Dis Esophagus. 2007;20(6):455-460.
2. Hanneman K, Newman B, Chan F. Congenital Variants and Anomalies of the Aortic Arch. Radiographics. 2017;37(1):32-51.
3. Arakoni R, Merrill R, Simon EL. Foreign body sensation: A rare case of dysphagia lusoria in a healthy female. Am J Emerg Med. 2018;36(11):2134.e1-e2134.e2.
4. Asherson N. David Bayford. His syndrome and sign of dysphagia lusoria. Ann R Coll Surg Engl. 1979;61(1):63-67.
5. Janssen M, Baggen MGA, Veen HF, Smout AJPM. Dysphagia lusoria: clinical aspects, manometric findings, diagnosis, and therapy. Am J Gastroenterol. 2000;95(6):1411-1416.
6. Colon V, Grade A, Pulliam G, Johnson C, Fass R. Effect of doses of glucagon used to treat food impaction on esophageal motor function of normal subjects. Dysphagia. 1999 Winter;14(1):27-30. doi: 10.1007/PL00009581. PMID: 9828271.
7. Steele CM, Alsanei WA, Ayanikalath S, et al. The influence of food texture and liquid consistency modification on swallowing physiology and function: a systematic review. Dysphagia. 2015;30(1):2-26.
8. Bogliolo G, Ferrara M, Masoni L, Pietropaolo V, Pizzicannella G, Miscusi G. Dysphagia lusoria: proposal of a new treatment. Surg Endosc. 1987;1(4):225-227.
9. Kieffer E, Bahnini A, Koskas F. Aberrant subclavian artery: surgical treatment in thirty-three adult patients. J Vasc Surg. 1994;19(1):100-109; discussion 110-111.
10. Klinkhamer AC. Het vastellen van aberrante arteriën in het mediastinum superius door middel van het oesophagogram. Published online 1962.
11. Triantopoulou C, Ioannidis I, Komitopoulos N, Papailiou J. Aneurysm of aberrant right subclavian artery causing Dysphagia lusoria in an elderly patient. AJR Am J Roentgenol. 2005;184(3):1030-1032.
12. Sitzman TJ, Mell MW, Acher CW. Adult-Onset Dysphagia Lusoria from an Uncommon Vascular Ring: A Case Report and Review of the Literature. Vasc
Endovascular Surg. 2009;43(1):100-102. Rogers AD, Nel M, Eloff EP, Naidoo NG. Dysphagia lusoria: a case of an aberrant right subclavian artery and a bicarotid trunk. ISRN Surg. 2011;2011:819295.
13. Bisognano JD, Young B, Brown JM, Gill EA, Fang FC, Zisman LS. Diverse Presentation of Aberrant Origin of the Right Subclavian Artery: Two Case Reports. Chest. 1997;112(6):1693-1697.