Endometriosis: A common cause of chronic abdominal pain in females
Jamison Williams, DO.
Jeremy Brown, DO.
Daniel Tritz, DO.
Ryan Essex, DO.
Bo Yon Choi, DO.
Donald von Borstel, DO.
Shelby Brown, DO
Jamison Williams, DO.
744 W 9th St
Tulsa, OK 74127
jamisondwilliams@gmail.com
Author’s Institution: Oklahoma State University Medical Center
Funding Associated with this manuscript: No funding was received to produce this manuscript.
Conflicts of Interest: No conflicts of interest to disclose with the production of this manuscript.
Abstract
Endometriosis is the implantation of uterine tissue outside of the uterus and is a common cause of chronic abdominal pain in females of reproductive age affected up to 15% of women with chronic abdominal pain.1 Due to the proposed etiology of disease by retrograde flow of uterine lining, it is conceivable for endometriosis to occur anywhere within the abdominal cavity, though the most common locations are the ovaries fallopian tubes, bladder, and rectosigmoid colon. A female of reproductive age presented for evaluation of an umbilical mass measuring approximately 3 x 3 cm. Contrast enhanced abdominal CT scan revealed multiple soft tissue densities throughout the abdomen and abdominal wall. Subsequent biopsy of the umbilical mass revealed endometrial tissue.
Key words: Endometriosis, abdominal pain, uterine fibroids, umbilical mass
Introduction
This case illustrates the typical clinical presentation and diagnostic evaluation in a patient with severe endometriosis.
Case Report
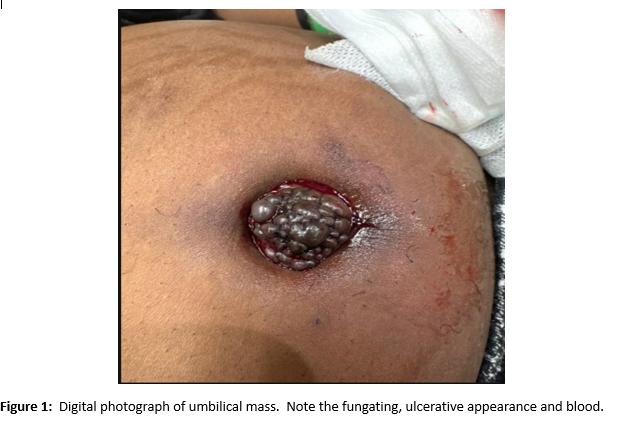
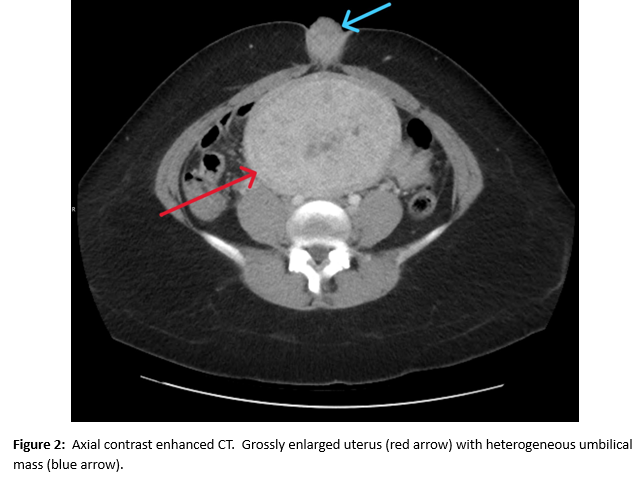
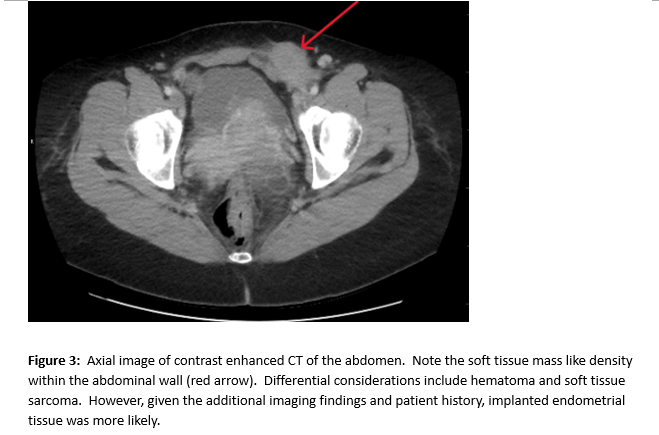
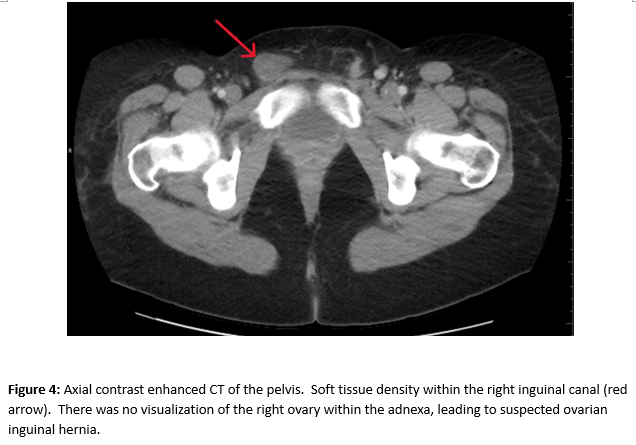
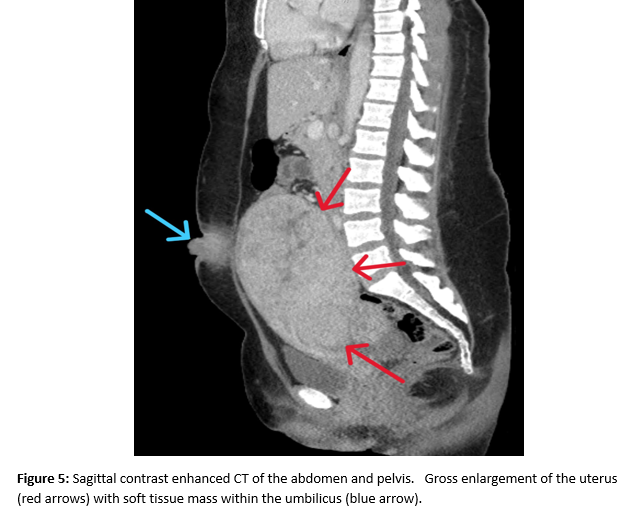
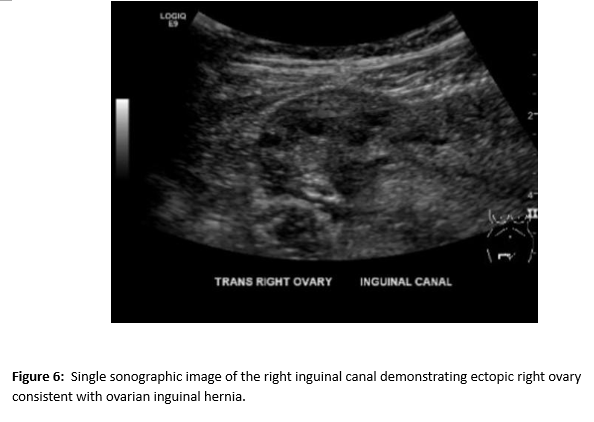
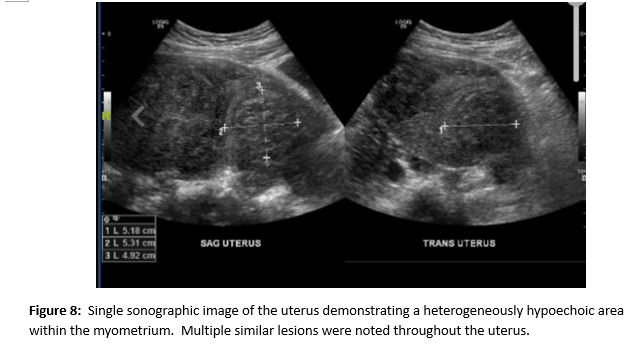
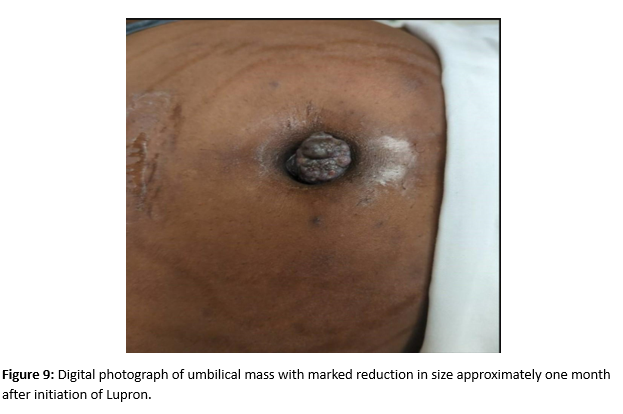
A female of childbearing age presented to the Emergency Department for evaluation of an umbilical mass. She reported the mass had slowly increased in size over several years and would bleed randomly (Figure 1). There was minimal associated pain with a longstanding history of pelvic pain and fullness. During her evaluation in the emergency department, she underwent a contrast enhanced CT of the abdomen and pelvis. Notable findings included a grossly enlarged uterus with multiple leiomyomas and a heterogeneously enhancing umbilical mass. Additionally, there were multiple soft tissue densities within the abdomen and abdominal wall and suspected herniation of the right ovary into the inguinal canal(Figures 2-5). Ob/Gyn was consulted from the emergency department.
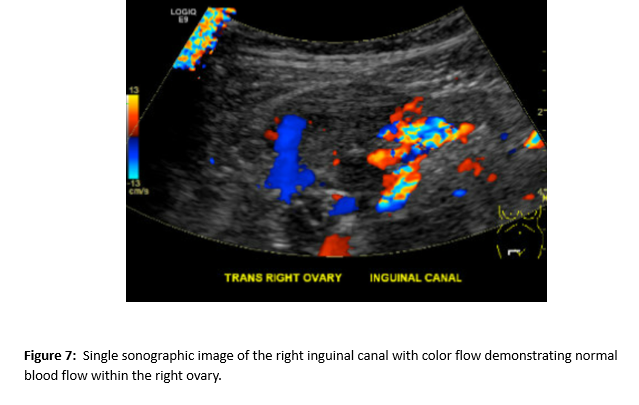
A follow up pelvic ultrasound demonstrated multiple uterine fibroids and the right ovary within the inguinal canal with normal blood flow (Figures 6 and 7). Speculum examination revealed healthy, pink vaginal tissue, nulliparous cervix, and moderate amount of blood in the vaginal blood secondary to menstruation. Biopsy samples were obtained of the umbilical mass as well as the endocervix and endometrium. Due to lack of current insurance coverage, the patient denied further inpatient workup. Histopathology returned on the umbilical mass which was positive for endometrial tissue and negative for malignancy. Endometrial and endocervical biopsies were both negative for malignancy. The patient followed up in the Ob/Gyn clinic where plans were made for future hysterectomy. Due to the gross enlargement of the uterus, the patient was not a candidate for laproscopic hysterectomy. However, the patient was agreeable to open hysterectomy with the possible addition of oophorectomy. The patient was initiated on Lupron in order to decrease the size of her uterus. At subsequent appointments approximately one month after initiation of Lupron, the patient did report a decrease in the size of her umbilical mass.
DISCUSSION
Endometriosis is a condition in which the presence of endometrial glands and stroma occurs outside of the uterine cavity. The presence of ectopic endometrial tissue is commonly demonstrated in the surrounding peritoneum and pelvic organs, but has been reported to have been seen in the kidneys, bladder, lungs, and brain1. It has been estimated that approximately 10 percent of reproductive-age females experience endometriosis, with peak prevalence in those from ages 25 to 35 2,3. The condition can commonly present asymptomatically, and be an incidental finding, whereas those with symptoms can experience a wide variety of complaints, including chronic abdominal and pelvic pain or pressure, dyspareunia, heavy menstrual bleeding, dysmenorrhea, and infertility. Symptoms can extend beyond the pelvic organs, and present as unusual symptoms such as bowel and bladder dysfunction, pleuritic chest pain, low back pain, and even chronic fatigue. 4
The theory behind development of endometriosis is varied, but the most widely accepted would be the theory of retrograde menstruation, or “Sampson’s Theory of Retrograde Menstruation”. 5 This proposed etiology theorizes that endometriosis results from the retrograde movement of endometrial cells and debris through the fallopian tubes during menstruation.5 This theory has been supported by the correlation between obstructive abnormalities, such as cervical stenosis or imperforate hymen, and the incidence of endometriosis. 6 However, in one study, it was estimated that up to 90 percent of females without obstructed fallopian tubes can experience retrograde menstruation, and the prevalence of endometriosis does not follow the statistical prevalence of retrograde menstruation .7
Other theories behind the development of endometriosis include the Coelomic Metaplasia Theory, which proposes that the ectopic endometrial tissue can develop from metaplasia of the tissues lining the peritoneum, often from a hormonal, infectious, or environmental stimulus. 8 Finally, the Lymphovascular Metastasis Theory follows that endometrial tissue can spread to aforementioned distant sites via hematogenous or lymphatic spread. 9
Regardless of the origin of the ectopic endometrial tissue and theory that one ascribes to, the image findings are the same. Ultrasound and Magnetic Resonance Imaging (MRI) are the two
recommended diagnostic studies recommended by the European Society for Human Reproduction and Embryology. MRI has been reported to have high sensitivity and specificity, 90% and 91%, respectively, whereas Transvaginal Ultrasound has demonstrated above 90% for both in the diagnosis of deep endometriosis, dependent on assessment of anterior and posterior compartments. 10,11.
Sonographic appearance of an endometrial nodule tend to be solid, hypoechoic, and irregular in
shape. They may also contain small cystic inclusions. Occasionally, these nodules may have small, echogenic foci and will display little to no blood flow when viewed on color Doppler. For deep endometriosis, several characteristic ultrasound findings are described: an anteverted-retroflexed uterus may often be seen, presence of hydrosalpinx in the fallopian tubes, thickening of the vaginal wall, obliteration of the Pouch of Douglas, and scattered nodules in various locations. 12
When compared to ultrasound, MRI has greater specificity overall for endometrial diagnosis.13 There are several limitations to the imaging modality, including the increased difficulty of detection of non- pigmented lesions due to lack of hyperintensity on T1 imaging. In addition, small foci can vary in their signal intensity and can appear similarly to normal tissue.
Despite these drawbacks, MRI is very useful in the diagnosis of endometriosis. The typical imaging appearance of endometriosis on MRI include small, solid lesions that may appear hyperintense on T1 and hypointense on T2 sequences. 14 Adhesions and fibrosis are isointense to pelvic muscle on both T1 and T2, in addition to distorted normal anatomy, hydrosalpinx, and potentially loculated fluid collections. Endometriomas demonstrate low signal intensity on T1 and T2 sequences [14]. Vaginal wall thickening can be appreciated, along with loss of hypointense signaling on T2 within the vaginal canal.14 Similar to ultrasound, nodules throughout the aforementioned locations may also be detected with MRI, with the exception of the rectum, which has lower sensitivity due to rectal content artifacts. 15
Overall, both MRI and ultrasound are the primary imaging modalities used in the diagnosis of endometriosis and also when evaluating treatment efficacy in these patients.
References
1. Pritts EA, Taylor RN. An evidence-based evaluation of endometriosis-associated infertility. Endocrinology and Metabolism Clinics of North America. 2003;32(3):653–667.
2. Shafrir AL, Farland LV, Shah DK, Harris HR, Kvaskoff M, Zondervan K, Missmer SA. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol. 2018;51:1.
3. Vercellini P, ViganòP, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocinol. 10; 5: 261-275.
4. Sinaii N, Plumb K, Cotton L, Lambert A, Kennedy S, Zondervan K, Stratton P. Differences in characteristics among 1,000 women with endometriosis based on extent of disease. Fertil Steril. 2008;89(3):538.
5. Sampson JA. Heterotopic or misplaced endometrial tissue. American Journal of Obstetrics and Gynecology. 1925;10(5):649–664.
6. Burney RO, Giudice L. Pathogenesis and pathophysiology of endometriosis. Fertility and Sterility. 2012;98:511–519.
7. Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol.1984 Aug;64(2):151-4.
8. Gruenwald P. Origin of endometriosis from the mesenchyme of the coelomic walls. Am J Obstet Gynecol. 1942;44:474.
9. Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927;3:109.
10. Bazot M, Darai E, Hourani R et-al. Deep pelvic endometriosis: MR imaging for diagnosis and prediction of extension of disease. Radiology. 2004;232 (2): 379-89.
11. Friedman H, Vogelzang RL, Mendelson EB et-al. Endometriosis detection by US with laparoscopic correlation. Radiology. 1985;157: 217-20.
12. Kinkel K, Frei KA, Balleyguier C, Chapron C. Diagnosis of endometriosis with imaging: review. European Radiology. 16, 285-298.
13. Woodward PJ, Sohaey R, Mezzetti TP. Endometriosis: radiologic-pathologic correlation. Radiographics. 21;1: 193-216.
14. Foti PV, Farina R, Palmuicci, et al. Endometriosis: clinical features, MR imaging findings and pathologic correlation. Insights Imaging. 9: 149-172.
15. Kinkel K, Chapron C, Balleyguier C et-al. Magnetic resonance imaging characteristics of deep endometriosis. Hum. Reprod. 14;4: 1080-1086.