Efficacy and safety evaluation of chloroquine treatment in patients with COVID-19: an observational multicenter cohort study
Guiqing He., Department of Infectious Diseases, Wenzhou Sixth People’ s Hospital, Wenzhou Central Hospital Medical Group, Affiliated Dingli Clinical Institute of Wenzhou Medical University, Wenzhou 325000, China; Infectious Diseases Laboratory, Wenzhou Sixth People’ s Hospital, Wenzhou Central Hospital Medical Group, Affiliated Dingli Clinical Institute of Wenzhou Medical University, Wenzhou 325000, China.
Jing Cai., Department of Comprehensive Medicine, Wenzhou Sixth People’ s Hospital, Wenzhou Central Hospital Medical Group, Affiliated Dingli Clinical Institute of Wenzhou Medical University, Wenzhou 325000, China.
Lingyan Fan., Department of Acute Infection, Hwa Mei Hospital, University of Chinese Academy of Sciences, Ningbo 315000, China.
Peipei Fang., Department of Infectious Disease, the Second Affiliated Hospital & Yuying Children’ s Hospital of Wenzhou Medical University, Wenzhou 325027, China.
Min Zhou., Department of Infectious Diseases, Xinyu People’s Hospital, Xinyu 338000, China.
Wenjie Sun., Center for Rural Health, Oklahoma State University Center for Health Sciences;
Jing Wu., Department of Infectious Diseases, Huashan Hospital, Fudan University, Shanghai 200040, China
These authors contribute equally, Correspondence author
ABSTRACT
Background There is no certified effective therapeutic against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) currently. Chloroquine has been shown to inhibit SARS-CoV-2 replication in vitro and used to treat pneumonia causing by COVID-19. We evaluated the efficacy and safety of co-treatment with chloroquine and antiviral agents (vs. antiviral agents alone) in patients with COVID-19 from multiple hospitals in Zhejiang, China.
Methods This retrospective study included 251 patients hospitalized with confirmed COVID-19 who were grouped and analyzed based on whether they were administered the antiviral drugs: lopinavir/ritonavir, interferon alpha-2b, and arbidol alone (control group) or in combination with chloroquine (treatment group). The main primary outcome was SARS-CoV-2 RNA conversion time.
Results A total of 141 patients with confirmed COVID-19 were finally included in this study. There was no significant difference in conversion time of SARS-CoV-2 RNA between the chloroquine and no chloroquine groups in the overall and subgroup analyses (ps > 0.05). There were no significant differences in temperature recovery, computed tomography (CT) absorption ratio, and blood routine dynamics between the two groups (ps > 0.05). Although more adverse events were found in the chloroquine group, no severe adverse events were observed.
Conclusions Our study showed no significant acceleration in viral RNA clearance or clinical outcome improvement after the addition of chloroquine adding to the treatment of COVID-19. No serious safety events were observed in patients who received chloroquine.
Keywords COVID-19, pneumonia, chloroquine, efficacy, safety
INTRODUCTION
Coronavirus disease 2019 (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is currently a pandemic, with over 142 million confirmed cases in 215 countries, areas, or territories worldwide and over 3 million deaths according to the World Health Organization (WHO), as of April 21, 20211. To date, there are no effective drugs or treatments against SARS-CoV-2 and more than 5,420 ongoing clinical trials have been registered to investigate potential treatments to combat COVID-192. Chloroquine and its derivative hydroxychloroquine have been used as off-label antiviral drugs for the treatment of COVID-19. Chloroquine sulfate was approved for use in treating COVID-19 patients according to the "Guidance for Coronavirus Disease 2019: Prevention, Control, Diagnosis, and Management" from the National Health Commission on February 18 as an antiviral drug3. However, scientists and researchers still doubt the effectiveness of chloroquine and hydroxychloroquine in the treatment of COVID-194. Nevertheless, on March 28, the US Food and Drug Administration (FDA) issued an Emergency Use Authorization to allow hydroxychloroquine sulfate and chloroquine phosphate to be used for certain hospitalized patients with COVID-195. Although both chloroquine and hydroxychloroquine have shown potent activity against SARS-CoV-2 in vitro6, this approval is very unusual because of the lack of sufficient systemic clinical evidence in the review process. This development could be partly attributed to the emergency status of the COVID-19 pandemic.
Chloroquine, a classic medication for malaria, acts through the endocytic and secretory pathways to kill the parasite7. Vitro experiments show that chloroquine inhibits the replication of SARS-CoV-28. Chloroquine has the potential to be a specific medication to prevent and control the pandemic and, in particular, its low cost makes it economically attractive for countries or areas that lack sufficient medical resources.
A recent published letter briefly reported the efficacy of chloroquine in the treatment of COVID-199, and a study of 22 COVID-19 patients claimed chloroquine (n=10) was superior to lopinavir/ritonavir (n=12) on the negative for the viral RNA10. However, the conclusion of the study in COVID-19 patients was not convincing due to the small sample size, inappropriate study design, and ambiguous outcomes7. Consequently, the disagreement generated by the laboratory work and clinical performance should be addressed11.
Evidence show the side effects of chloroquine including vomiting, abdominal pain, nausea, diarrhea, rash or itching, cough, and shortness of breath have been observed10. Moreover, ophthalmological concerns associated with this agent have been raised12. Previously, many medications that showed effectiveness were later withdrawn because of adverse reactions11. Hence, the efficacy and safety of chloroquine needs to be examined in a multicenter, clinical setting, with subjective outcomes in COVID-19 patients. This present study conducted under such conditions provides data that are expected to shed light on the efficacy and safety of chloroquine in COVID-19 patients.
METHODS
Study design and participants
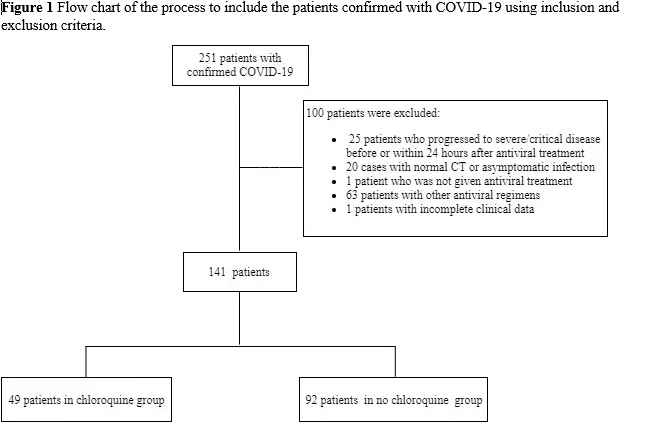
This multicenter, retrospective cohort study included 251 patients with confirmed COVID-19 admitted to Wenzhou Sixth People’s Hospital, Wenzhou Central Hospital Medical Group, and Huamei Hospital, Ningbo No. 2 Hospital, University of Chinese Academy of Sciences between January 17, 2020 and February 14, 2020. Patients were divided into two groups according to whether they were treated with the antiviral agents (lopinavir/ritonavir, interferon alpha-2b and arbidol) combined with or without chloroquine (treatment and control groups, respectively). We included patients who were aged ≥ 18 years, had a positive result for SARS-CoV-2 RNA, and had pneumonia confirmed using chest imaging. Exclusion criteria were as follows: patients (1) used other antiviral regimens or not administered antiviral treatment, (2) diagnosed with an asymptomatic infection or had normal chest computed tomography (CT) images upon admission, (3) with incomplete clinical data, and (4) who progressed to severe/critical illness before antiviral therapy or within 24 hours of treatment. According to the above criteria, 141 confirmed patients were included in the analysis (Figure 1) and the study was approved by the Ethics Commission of Wenzhou Central Hospital.
Outcomes
The primary endpoint was virological clearance time of nasopharyngeal or oropharyngeal swabs after antiviral therapy. Secondary endpoints were clinical outcomes in follow-up (rate of progression to severe/critical illness, time to normal body temperature in febrile patients, absorption rate of chest CT at week 2, blood routine dynamic changes within 2 weeks, days of hospital stay, and recurrent viral RNA positivity during follow-up period after discharge) and occurrence of adverse events
Statistical analysis
Continuous and categorical variables were compared between the chloroquine and no chloroquine groups. Continuous variables were described as medians (interquartile range [IQR]) and compared using the Mann-Whitney U test. Categorical variables were described as numbers (%) and compared using the chi-squared (χ²) test or Fisher’s exact test. Statistical analyses were performed using the statistical package for the social sciences (SPSS) software, version 19.0 (IBM Corp, Armonk, NY, USA), unless otherwise indicated. Comparison of RT-PCR RNA conversion time between the two groups are illustrated using GraphPad Prism 5.0 and tested using log-rank test. A two-sided α = 0.05 was considered statistically significant.
RESULTS
Demographic and baseline clinical characteristics
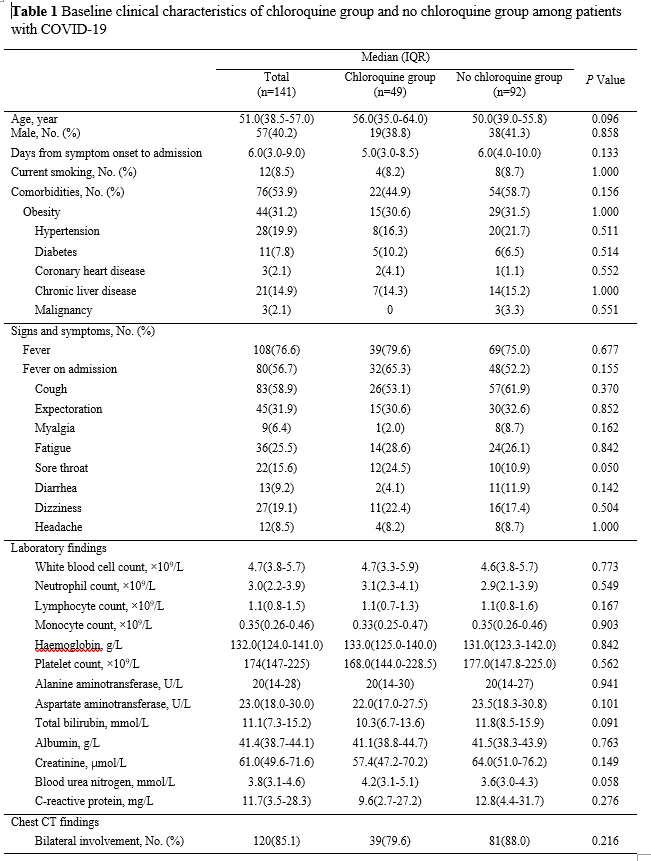
A total of 141 patients with confirmed COVID-19 were included in this study, comprising 49 and 92 in the treatment (chloroquine) and control (no chloroquine) groups, respectively. All patients received antiviral therapy with lopinavir/ritonavir, interferon alpha-2b, and arbidol immediately after admission. Chloroquine in the treatment group was co-administered with the antiviral agents and the median (IQR) time from symptom onset to treatment was 16 (12.5 to 18) days. Demographic and baseline clinical characteristics of these patients are summarized in Table 1. The median age of patients was 51 (38.5 to 57) years and 40.2% were men. The median interval time from symptom onset to admission was 6 (3 to 9) days. There were no significant differences between the two groups in demographic characteristics, baseline laboratory test results, and chest CT findings at enrollment (Table 1).
Effect of chloroquine in patients with COVID-19
Primary outcomes
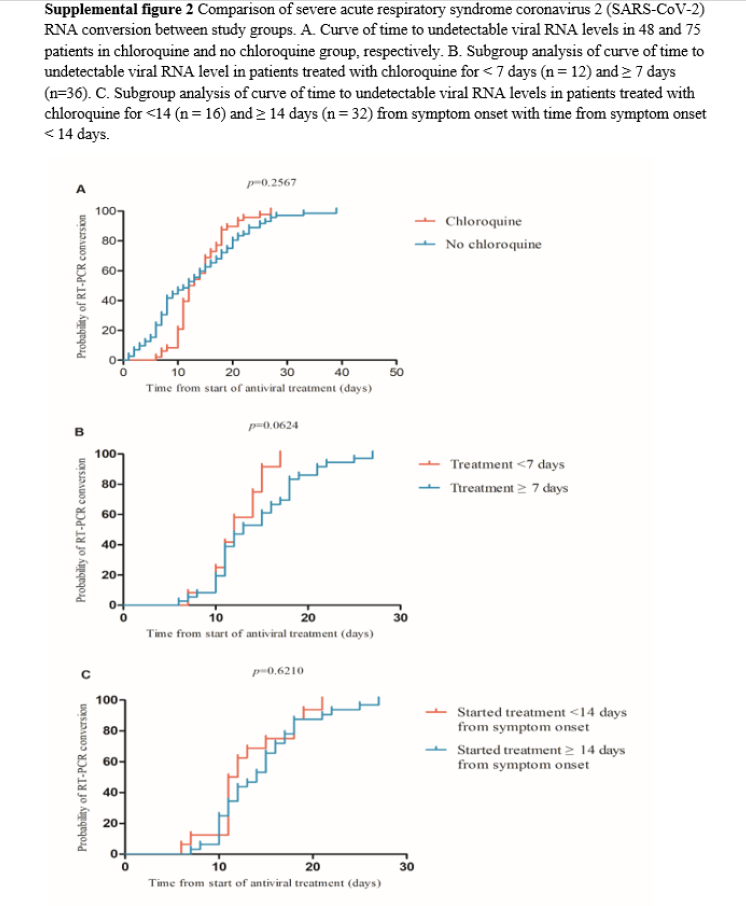
Among the 141 patients included in the study, 18 were excluded because they had incomplete dynamic viral RNA testing information and, consequently, 123 patients comprising 48 and 75 in the chloroquine and no chloroquine groups, respectively, were included in the final analysis (Figure 1). There were no significant differences between the two groups in conversion of SARS-CoV-2 RNA (p=0.257, Supplemental figure 2A, Table 2). The 48 patients in the chloroquine group were further divided into subgroups for analysis according to the duration of treatment with chloroquine and time from symptom onset when chloroquine was added to the treatment regimen. There was no significant difference in viral RNA conversion between a short (< 7 days) and long (≥ 7 days) treatment duration (p=0.062, Supplemental figure 2B). Following chloroquine treatment, the conversion of SARS-CoV-2 RNA in patients with time from symptom onset < 14 days was not significantly faster than that of the group with time from symptom onset ≥ 14 days (p=0.621, Supplemental figure 2C).
We further analyzed the difference in RT-PCR-detected RNA conversion after antiviral therapy between the two groups within 7 days from symptom onset. Among 123 patients, 41 with a
disease course longer than 7 days following admission were excluded and, subsequently, 82 patients comprising 33 and 49 in the chloroquine and no chloroquine group, respectively, were included in the final analysis. There was no significant difference in conversion of SARS-CoV-2 RNA between the chloroquine and no chloroquine group (p = 0.083, Supplemental figure 3A). The 33 patients in the chloroquine group were further divided into subgroups for analysis according to the duration treatment with chloroquine and time from symptom onset when chloroquine was added to the regimen (Figure 1). The conversion of SARS-CoV-2 RNA in the group treated with chloroquine for ≥ 7 days was not significantly faster than that in the group treated with chloroquine for < 7 days (p = 0.134, Supplemental figure 3B). There was no difference in the conversion time of SARS-CoV-2 RNA between the groups with time from symptom onset ≥ 14 days and < 14 days (p=0.804, Supplemental figure 3C).
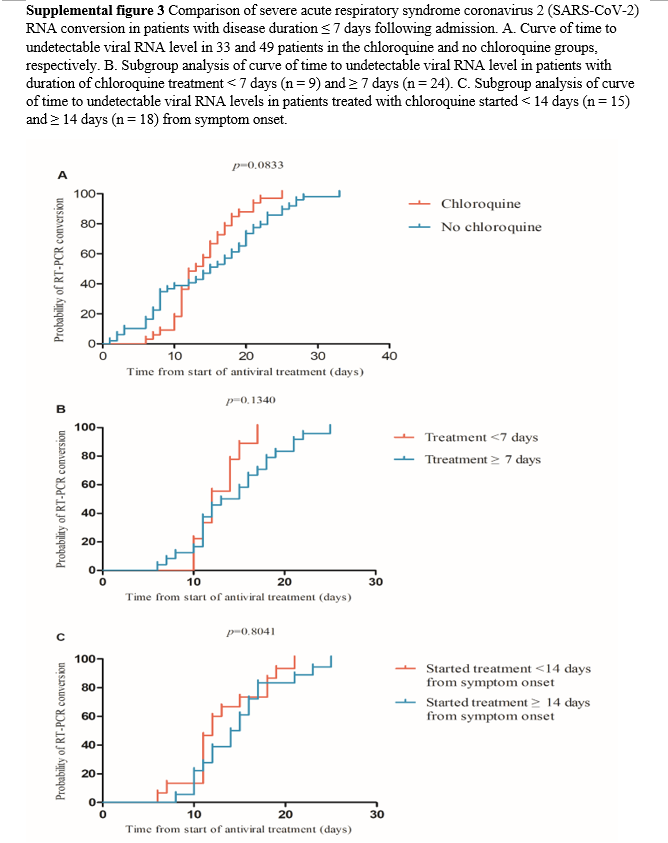
Secondary outcomes
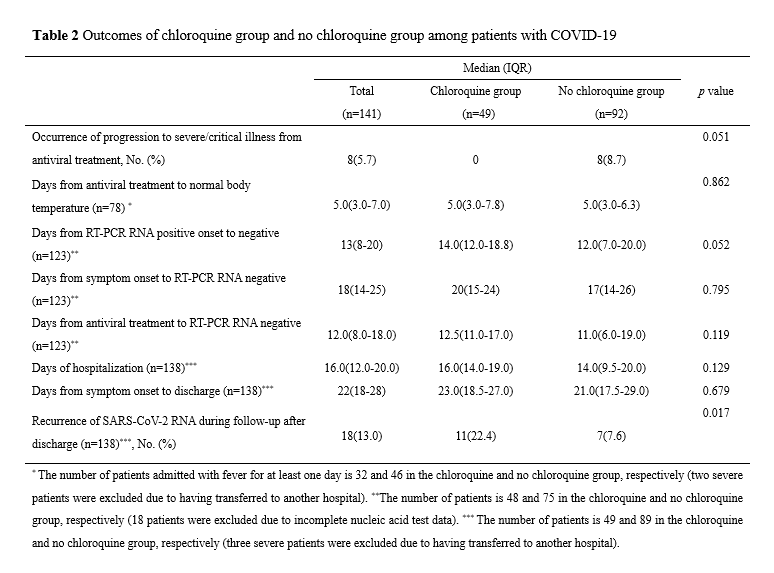
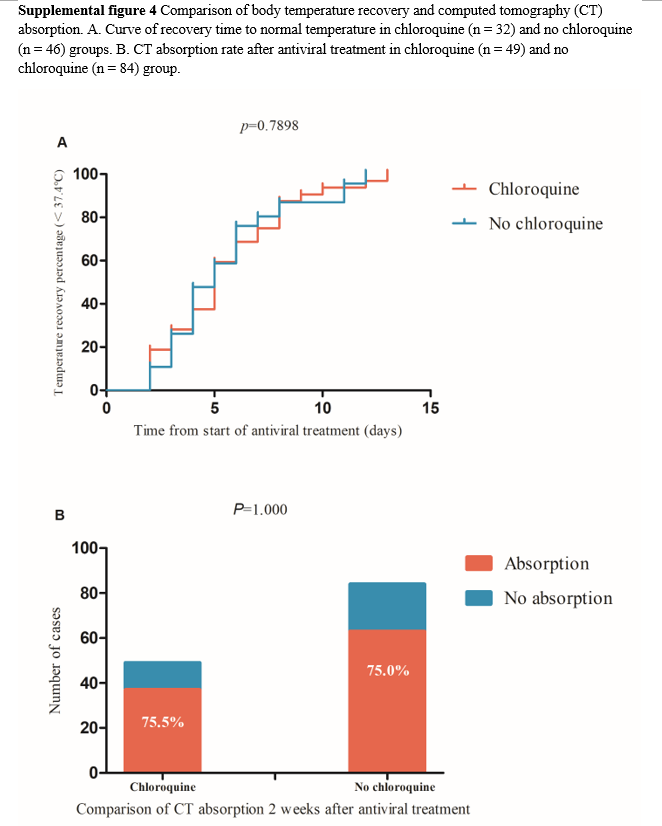
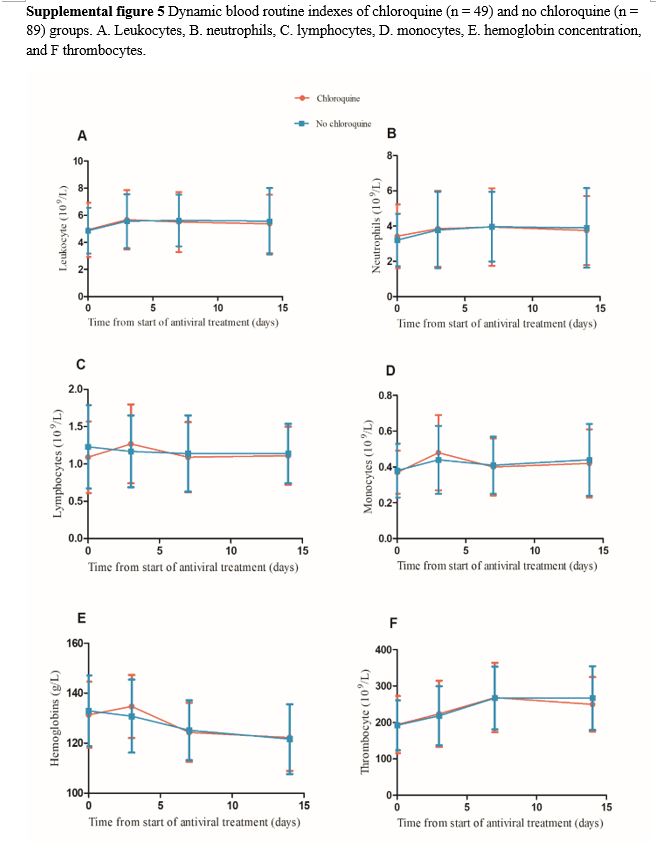
The occurrence of progression to severe or critical illness was higher in the no chloroquine group than in the chloroquine group, and there was a strong tendency towards a statistically significant difference between the two groups (8.7% vs 0%, p=0.051, Table 2). Furthermore, 80 of 141 patients had fever upon admission and 2 were excluded because they still had fever before they were transferred to another hospital. In addition, 78 patients were finally included in the analysis, comprising 32 and 46 in the chloroquine and no chloroquine groups, respectively. There was no significant difference between the two groups in proportion and the time from antiviral treatment to temperature recovery (Supplemental figure 4A) or in the length of hospitalization and the time from symptom onset to discharge. Recurrence of positive SARS-CoV-2 RNA in subsequent sample testing during follow-up period after discharge was significantly higher in the chloroquine group than in the no chloroquine group (22.4 % vs 7.6%, p = 0.017). Among the 141 patients, 8 with missing CT re-examination data 2 weekends after antiviral treatment were excluded and 133 patients were finally included in the comparative CT analysis, comprising 49 and 84 in the chloroquine and no chloroquine groups, respectively. There was no significant difference in the CT-detected absorption ratio between both groups (75.5% vs 75%, p =1.000, Supplemental figure 4B). Three patients transferred to another hospital were excluded and the blood routine dynamics were compared between 49 and 89 patients in the chloroquine and no chloroquine groups, respectively. There were no significant differences in the dynamic changes of leukocytes, neutrophils, lymphocytes, monocytes, thrombocytes, and hemoglobin concentration between the two groups (Supplemental figure 5).
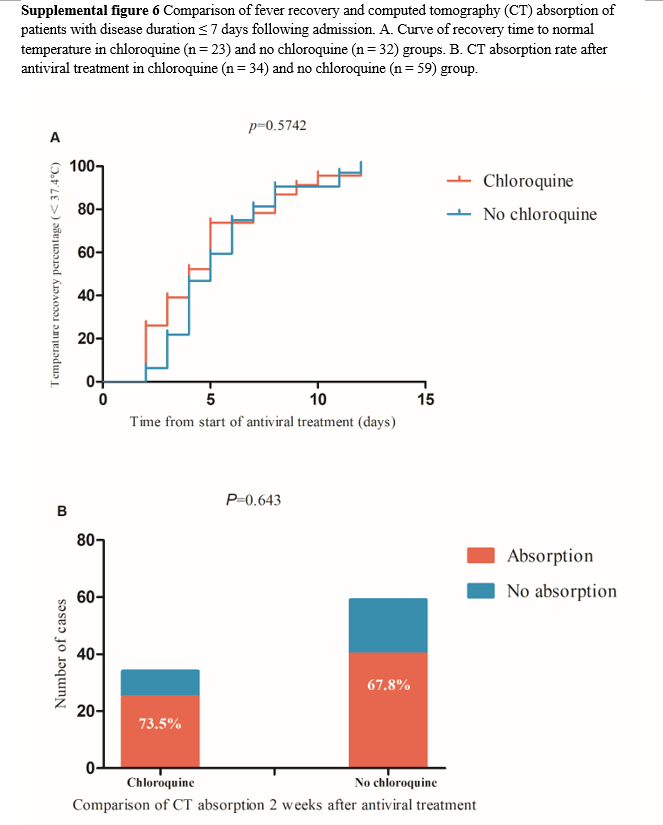
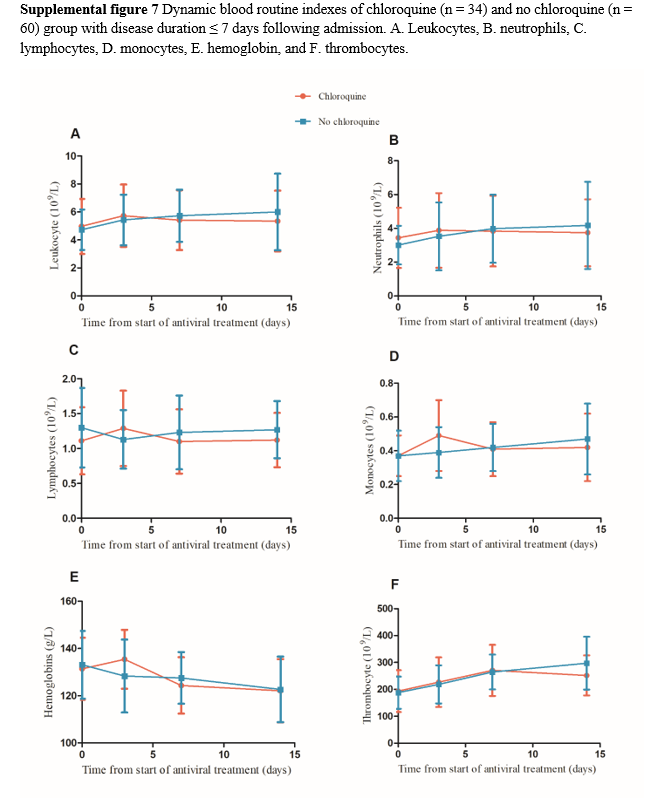
Among the 78 patients with fever, 13 were excluded because their time from symptom onset was > 7 days and 55 patients were finally included in the analysis, comprising 23 and 32 in the chloroquine and no chloroquine groups, respectively. There was no significant difference in the time from antiviral treatment to temperature recovery between the two groups (Supplemental figure 6A). Of 133 patients, 40 were excluded because their time from symptom onset was > 7 days, and 93 patients were finally included for comparative CT analysis, comprising 34 and 59 in the chloroquine and no chloroquine groups, respectively. The CT absorption in the chloroquine group was a slightly higher than that of the no chloroquine group, but there was no significant difference between the two groups (73.5% vs 67.8%, p = 0.643, Supplemental figure 6B). In the dynamic comparison of routine blood test results, patients with time from symptom onset > 7 days were excluded, and 94 cases were included in the analysis, comprising 34 and 60 in chloroquine and no chloroquine groups, respectively. No significant differences in the dynamic changes were found between the two groups (Supplemental figure 7).
Safety
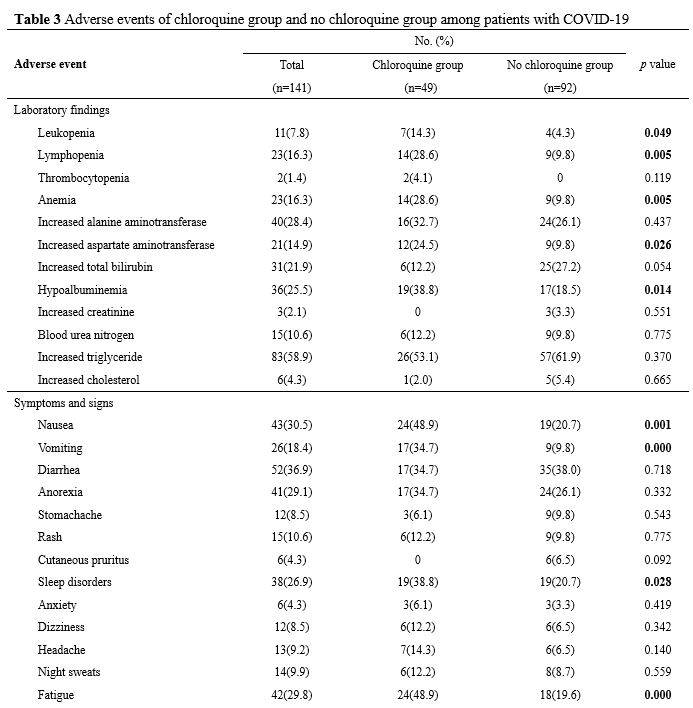
Potential adverse effects are compared in Table 3. Adverse events related to blood biochemistry and routine blood test including leukopenia, lymphocytopenia, anemia, elevated aspartate aminotransferase, and hypoalbuminemia were more common in the chloroquine group than in the no chloroquine group. However, the percentage of patients with other laboratory abnormalities was similar in the two groups. The incidence of gastrointestinal adverse events including nausea and vomiting were significantly higher in the chloroquine group than in the no chloroquine group. The incidence of other adverse events including sleep disorders, fatigue, and pharyngeal discomfort were also significantly higher in the chloroquine group than in the no chloroquine group. There were no differences in other symptoms and signs of adverse events between the two groups.
DISCUSSION
This observational study retrospectively analyzed COVID-19 patients treated with antiviral regimens with or without chloroquine, and our findings support that chloroquine was not associated with improvements of clinical outcomes. Our data did not show faster overall clearance of SARS-CoV-2 RNA. However, the subgroup analysis data may indicate that early use of chloroquine in patients within 1 week from symptom onset following admission may shorten the time of viral RNA clearance. Chloroquine was introduced into clinical use in the treatment of COVID-19 because it was verified to have antiviral activity against SARS-CoV-2 in vitro in recent studies8. However, that study was not the first to report the antiviral efficacy of
chloroquine and previous studies have demonstrated the in vitro or in vivo growth inhibition of several viruses by chloroquine, including SARS-CoV13, influenza A H5N114, and Zika virus15, enterovirus EV-A7116. These positive results have initiated numerous clinical trials including one investigating the treatment of chikungunya with chloroquine or placebo, and no improvement was observed in controlling the disease progression in chloroquine-treated patients17. Two clinical trials investigated chloroquine for the treatment of dengue virus (DENV) infection18. One study reported a longer duration of DENV viremia in patients treated with chloroquine19 while the other, which had a small sample size, showed no significant difference in disease duration or degree and days of fever in patients administered20. To date, no previous clinical trials have provided sufficient evidence that chloroquine has antiviral effects on other acute viral infections in humans.
The COVID-19 pandemic has created a global emergency in the search for a treatment, but there is a lack of standard randomized controlled trials with a large enough sample size. Studies showing benefits of hydroxychloroquine in patients with COVID-19 have been reported by different groups including one conducted in France with 16 patients treated with hydroxychloroquine and 6 with azithromycin add-on, which showed a significant reduction of viral carriage21. However, the result was soon questioned due to the small sample size, lack of randomized control, and other confounding factors. In a randomized clinical trial comparing 31 hydroxychloroquine-treated to 31 control COVID-19 patients in Wuhan, a faster clinical recovery was observed in treated patients22. Subsequent studies have raised doubts about the effectiveness of hydroxychloroquine including an observational study with 811 patients treated with hydroxychloroquine in New York, which did not demonstrate a reduced or increased risk of intubation or death23. A small sample-sized retrospective study also found that hydroxychloroquine was associated with a slower viral clearance in mild to moderate COVID-19 patients24. A multicenter observational study with chloroquine was recently conducted in 12 hospitals in Guangdong and Hubei of China25. The results showed that although the interval time from symptom onset to treatment initiation was > 7 days, patients in the chloroquine group (n = 197) experienced a significantly faster and higher rate of viral conversion to a negative result than those of the non-chloroquine group (n=176)25. However, our study only showed a possibility of viral clearance promotion with the early use of chloroquine, but not in all patients treated with the agent as a later add-on.
More and more evidence were added against the use of (hydroxy)chloroquine in hospitalized patients with COVID-19. Early in May, Chinese clinicians published that hydroxychloroquine did not result in a significantly higher probability of negative conversion than standard of care alone in patients admitted to hospital with mild to moderate COVID-1926. A study in UK indicated among patients hospitalized with COVID -19, those who received hydroxychloroquine did not have a lower incidence of death at 28 days than those who received usual care. A study in the Netherlands analyzed data of COVID-19 patients treated in nine hospitals, and found mortality was not significantly different in hospitals that routinely treated patients with (hydroxy)chloroquine compared with hospitals that did not27. Another study showed hydroxychloroquine did not prevent illness compatible with COVID-19 or confirmed infection when used as postexposure prophylaxis within 4 days after exposure28.
Both chloroquine and hydroxychloroquine are well tolerated in the clinical treatment of malaria and rheumatic diseases. The severe adverse effects of these agents include prolongation of the
QTc interval, hypoglycemia, neuropsychiatric effects, and idiosyncratic hypersensitivity reactions29. Attention has been focused on the safety of chloroquine and hydroxychloroquine in severely or critical ill COVID-19 patients. An association was identified between increased mortality and hydroxychloroquine treatment of patients in a retrospective analysis30 and one of the confounding was that hydroxychloroquine was more likely to be used in patients with serious diseases. Another study evaluating high- and low-dose chloroquine found that a higher dose was associated with lethality, and a higher incidence of QTc interval prolongation31. Consistent with other studies according to recent systematic reviews32, our study demonstrated that no severe adverse events were observed, although more adverse effects were found in the chloroquine group.
Our study has some limitations, which are worth mentioning. As a retrospective observational study, it lacked randomization. Most patients had a moderate condition, which did not develop to severe illness, so we could not evaluate if chloroquine could reduce the disease progression. Moreover, chloroquine was added to the various regimens at different times during the progression of the disease and was more likely to be prescribed to patients with longer SARS-CoV-2 viral RNA positive status. Therefore, we attempted to minimize possible confounding factors by conducting numerous subgroup analyses in our study.
CONCLUSIONS
Our study investigated chloroquine therapy in a multicenter cohort of patients hospitalized with COVID-19. Chloroquine did not demonstrate significant efficacy in clearing SARS-CoV-2 viral RNA or improving clinical symptoms of patients. The potential of chloroquine to shorten the conversion time of SARS-CoV-2 viral RNA to a negative status in patients who are treated early following disease onset should be further investigated in randomized studies.
DECLARATIONS
This study was approved with written consent by the Ethic Committee of Wenzhou Central Hospital (No. L2020-04-045). Written consents were obtained from the patients.
Competing interests
The authors have no conflict of interest to disclose.
REFERENCES
1. World Health Organization. WHO Coronavirus (COVID-19) Dashboard Overview. https://covid19.who.int/. Accessed April 21, 2021.
2. ClinicalTrials.gov. https://clinicaltrials.gov/. Accessed April 21, 2021.
3. National Health Commission. Interpretation of the Sixth Edition of the Guidance for COVID-19: Prevention, Control, Diagnosis, and Management. http://www.nhc.gov.cn/xcs/fkdt/202002/54e1ad5c2aac45c19eb541799bf637e9.shtml.
4. Lenzer J. Covid-19: US gives emergency approval to hydroxychloroquine despite lack of evidence. BMJ. 2020;369:m1335.
5. Ferner RE, Aronson JK. Chloroquine and hydroxychloroquine in covid-19. BMJ. 2020;369:m1432.
6. Emergency Use Authorization. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization.
7. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269-271.
8. Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16.
9. Engel S, Heger T, Mancini R, et al. Role of endosomes in simian virus 40 entry and infection. J Virol. 2011;85(9):4198-4211.
10. Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72-73.
11. Huang M, Tang T, Pang P, et al. Treating COVID-19 with Chloroquine. J Mol Cell Biol. 2020;12(4):322-325.
12. Marmor MF. COVID-19 and Chloroquine/Hydroxychloroquine: is there Ophthalmological Concern? Am J Ophthalmol. 2020;213:A3-A4.
13. Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323(1):264-268.
14. Yan Y, Zou Z, Sun Y, et al. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res. 2013;23(2):300-302.
15. Li C, Zhu X, Ji X, et al. Chloroquine, a FDA-approved Drug, Prevents Zika Virus Infection and its Associated Congenital Microcephaly in Mice. EBioMedicine. 2017;24:189-194.
16. Tan YW, Yam WK, Sun J, Chu JJH. An evaluation of Chloroquine as a broad-acting antiviral against Hand, Foot and Mouth Disease. Antiviral Res. 2018;149:143-149.
17. De Lamballerie X, Boisson V, Reynier JC, et al. On chikungunya acute infection and chloroquine treatment. Vector Borne Zoonotic Dis. 2008;8(6):837-839.
18. Tricou V, Minh NN, Van TP, et al. A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS Negl Trop Dis. 2010;4(8):e785.
19. Borges MC, Castro LA, Fonseca BA. Chloroquine use improves dengue-related symptoms. Mem Inst Oswaldo Cruz. 2013;108(5):596-599.
20. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949.
21. Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv 2020.04.26.20081059.
22. Geleris J, Sun Y, Platt J, et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020;382(25):2411-2418.
23. Mallat J, Hamed F, Balkis M, et al. Hydroxychloroquine is associated with slower viral clearance in clinical COVID-19 patients with mild to moderate disease: A retrospective study. medRxiv 2020.04.27.20082180.
24. Huang M, Li M, Xiao F, et al. Preliminary evidence from a multicenter prospective observational study of the safety and efficacy of chloroquine for the treatment of COVID-19. medRxiv 2020.04.26.20081059.
25. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849.
26. Peters EJ, Collard D, Van Assen S, et al. Outcomes of persons with coronavirus disease 2019 in hospitals with and without standard treatment with (hydroxy)chloroquine. Clin Microbiol Infect. 2020.
27. Boulware DR, Pullen MF, Bangdiwala AS, et al. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N Engl J Med. 2020;383(6):517-525.
28. Juurlink DN. Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection. CMAJ. 2020;192(17):E450-E453.
29. Magagnoli J, Narendran S, Pereira F, et al. Outcomes of Hydroxychloroquine Usage in United States Veterans Hospitalized with COVID-19. Med (N Y). 2020.
30. Borba MGS, Val FFA, Sampaio VS, et al. Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial. JAMA Netw Open. 2020;3(4):e208857.
31. Peng H, Chen Z, Wang Y, et al. Systematic Review and Pharmacological Considerations for Chloroquine and Its Analogs in the Treatment for COVID-19. Front Pharmacol. 2020;11:554172.
32. Yao X, Yan X, Wang X, et al. Population-based meta-analysis of chloroquine: informing chloroquine pharmacokinetics in COVID-19 patients. Eur J Clin Pharmacol. 2020.