Microcytic Anemia in the Setting of Helminth Infection
Kate Riley, DO., Department of Internal Medicine, Oklahoma State University Medical Center, Tulsa Oklahoma
Fernando Magana, DO, Oklahoma State University Department of Gastroenterology, St. Francis Hospital, Tulsa, Oklahoma
Abigail Carroll, DO, Department of Internal Medicine, Oklahoma State University Medical Center, Tulsa Oklahoma
William Nowlin, DO, Department of Internal Medicine, University of Oklahoma, Tulsa, Oklahoma
Andrew Harris, DO, Department of Internal Medicine, Oklahoma State University Medical Center, Tulsa Oklahoma
Vasudevan Raghuraman, MD., Oklahoma State University Department of Gastroenterology, St. Francis Hospital, Tulsa, Oklahoma
Corresponding Author:
Abigail Carroll, DO
Address: 11009 N. 154th E. Ave. Owasso, Oklahoma 74055
Email: ahollihan97@gmail.com
Phone: 918-740-2515
Funding: No funding provided
Disclosures: No conflicts of interest
Abstract
Parasitic infections are endemic to developing countries due to poverty, poor water hygiene, and inadequate sanitation systems; however, the incidence is increasing in the United States. Parasitic infections can cause anemia, malnutrition, growth impairment, developmental and physical delays. Here, we present the case of a 27-year-old male who presented with profound iron deficiency anemia due to presumed Ascaris lumbricoides infection in the colon and Hookworm infection in the duodenum. He was treated with a one-time dose of Albendazole 400 mg. To help combat the prevalence and morbidity associated with helminth infections, mass drug administration (MDA) programs and clean water initiatives have been developed. There is still a significant need for ongoing assistance programs and research to look for interventions to help eradicate helminth infections. Our case helps highlight to providers the importance of evaluation for parasitic infections in non-endemic regions as a potential source of anemia.
Key Words: Hookworm, Roundworm, microcytic anemia, Albendazole therapy, Helminth infection
Introduction
Anemia is characterized by an inappropriately low hemoglobin level, which in turn decreases the oxygen carrying capacity of red blood cells1. Anemia can cause a constellation of symptoms, including shortness of breath, lightheadedness, fatigue, leg cramps, and hemodynamic compromise if severe. Iron deficiency anemia is the most common cause of anemia, whether from poor oral intake of iron, or chronic blood loss. There are many other causes of anemia ranging from chronic inflammation, kidney disease, and various nutrient deficiencies. Parasitic infections can also be an unusual, but devastating cause of anemia. Parasitic infections are most commonly contracted from the soil and are estimated to affect approximately 24% of the world’s population2. Parasitic infections are endemic to developing countries and are associated with poverty and poor environmental hygiene1. The highest prevalence of these infections is reported in sub-Saharan Africa, China, South America and Asia2. These infections are transmitted via eggs, present in human feces, that contaminate the soil in areas with poor sanitation structure. The eggs may then be consumed on poorly washed vegetables or ingested by children playing in the infected soil. Adult worms then hatch and inhabit the intestine where thousands of parasitic eggs are produced daily; those eggs are then shed in feces2. The main species of helminths that infect people include: the roundworm (Ascaris lumbricoides), the whipworm (Trichuris trichiura) and hookworms (Necator americanus and Ancylostoma duodenale)2. According to the Centers for Disease Control (CDC), there are still many parasites that are endemic within small pockets of the United States—most commonly in areas of poverty and poor sanitation3. Parasitic infections are a serious cause of morbidity in those affected. The helminths feed on the host’s tissues leading to chronic blood loss, protein loss, malabsorption of nutrients and chronic malnutrition2. In severe cases, some experience impaired growth, developmental and physical delay.
Case Presentation
The patient is a 27-year-old male with no pertinent past medical or surgical history that initially presented to the emergency department with severe anemia. He was evaluated by his primary care provider after five months of frontal headache and fatigue. Laboratory evaluation obtained by his primary care provider revealed significant anemia requiring prompt evaluation by the emergency department.
Upon arrival, the patient was afebrile, normotensive, and in no acute distress. He denied any history of anemia or previous blood transfusions, melena, hematochezia, or hematemesis. He reported taking ibuprofen up to three times daily since symptom onset. Laboratory evaluation was significant for hemoglobin 4.5, mean corpuscular volume 64.2, platelets 741, and white blood cells 3.9. Hemoccult was negative and two units of packed red blood cells were transfused. Computed tomography (CT) of the head without contrast was negative for acute intracranial processes.
Additional laboratory studies were significant for ferritin 2, iron level 13, and elevated reticulocytes with normal LDH and haptoglobin levels, confirming severe iron deficiency anemia (IDA) causing reactive thrombocytosis. Further history was obtained and revealed moderate alcohol consumption (7 drinks weekly) and recent emigration from Guatemala. With additional risk factors for gastrointestinal cause of severe IDA, oral Protonix was initiated, anticoagulation was not given for thrombocytopenia and gastroenterology was consulted for endoscopy.
Esophagogastroduodenoscopy (EGD) revealed LA grade B reflux esophagitis, mild non-reactive gastritis, and multiple live hookworms within the duodenal bulb, first and second portions of the duodenum (Figure 1 and 2).
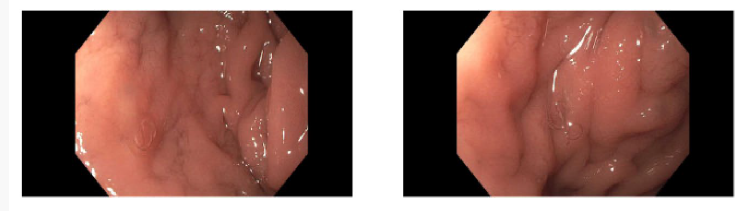
Figure 1&2.Endoscopic evaluation revealing live hookworms within the second portion of the duodenum.
Colonoscopy was significant for live roundworms (presumed Ascaris lumbricoides) within the cecum. (Figure 3). A specimen was biopsied via suction and sent to pathology for evaluation. The patient was treated with Albendazole 400mg.
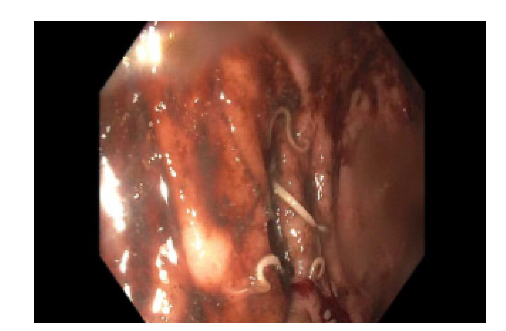
Figure 3. Endoscopic evaluation of the cecum revealing multiple roundworms.
After transfusion of two units of packed red blood cells and several days of intravenous iron replacement, the patient’s hemoglobin levels remained stable. He was given an additional dose of Albendazole 200 mg upon discharge and was instructed to follow up with his primary care physician to ensure infection eradication.
Discussion
This case describes a young patient with profound iron deficiency anemia due to a helminth infection. It should be noted that it is unknown when this patient emigrated to the United States and therefore difficult to ascertain how long he had been infected. This raises the question of how long a patient must be infected to start exhibiting signs of anemia. According to the Centers for Disease Control (CDC), most immigrants with parasitic infections are not diagnosed for greater than 5 years after emigration to the United States4. However, there is more research to be done to be able to ascertain the time frame when parasitic infection should be higher on the differential as a source of anemia in patients who emigrated from endemic countries. The patient was ultimately treated with a single dose of Albendazole 400 mg for presumed Ascaris lumbricoides infection in the colon and Hookworm infection in the duodenum. Albendazole’s active metabolite, albendazole sulfate, works to impair glucose metabolism in intestinal helminths, ultimately depleting their ATP production and causing energy depletion, immobilization, and death5. Currently, efforts to decrease the burden of intestinal helminth infections include mass drug administration (MDA) of anti-helminth medications to children living in endemic areas, as well as improving water hygiene and sanitation6. The three most common types of soil-transmitted helminths– the roundworm (Ascaris lumbricoides), the whipworm (Trichuris trichiura) and hookworms (Necator americanus and Ancylostoma duodenale), are susceptible (in varying degrees) to the benzimidazole drug class, making albendazole and mebendazole the most commonly used medications in MDA programs6. Although MDA programs have shown benefit in reducing prevalence of helminthic infections, they must be paired alongside clean water initiatives and improvement in sanitation. There is still much work to be done to decrease the burden and overall morbidity associated with these
parasitic infections. Future research could be aimed at possible vaccinations or other medications to assist in helminth eradication.
References
Gopalakrishnan S, Eashwar VMA, Muthulakshmi M, Geetha A. Intestinal parasitic infestations and anemia among urban female school children in Kancheepuram district, Tamil Nadu. Journal of Family Medicine and Primary Care. 2018;7(6):1395. doi:https://doi.org/10.4103/jfmpc.jfmpc_89_18
World Health Organization: WHO. Soil-transmitted helminth infections. Who.int. Published March 14, 2019. https://www.who.int/en/news-room/fact-sheets/detail/soil-transmitted-helminth-infections
Mathison, B., & Pritt, B. (2023, August). The landscape of parasitic infections in the United States. Modern Pathology. https://www.modernpathology.org/article/S0893-3952(23)00122-9/fulltext
Centers for Disease Control and Prevention. (2024, May 15). Intestinal parasites. Centers for Disease Control and Prevention. https://www.cdc.gov/immigrant-refugee-health/hcp/domestic-guidance/intestinal-parasites.html
Albendazole (Oral Route) Proper Use - Mayo Clinic. www.mayoclinic.org. https://www.mayoclinic.org/drugs-supplements/albendazole-oral-route/proper-use/drg-20061505?
Blair P, Diemert D. Update on Prevention and Treatment of Intestinal Helminth Infections. Current Infectious Disease Reports. 2015;17(3). doi:https://doi.org/10.1007/s11908-015-0465-x
Haque R. Human intestinal parasites. J Health Popul Nutr. 2007;25(4):387-391.