Pancreatic Schwannoma: A rare case in the setting of Diffuse B-cell Lymphoma and Follicular Lymphoma
Magana Herrera, Fernando D.O.; Department of Gastroenterology, Oklahoma State University, Tulsa,OK
Heavener, Trace D.O,MBA; Department of Gastroenterology, Oklahoma State University, Tulsa,OK
Sharma Haresh, Nisha M.D.; Department of Gastroenterology, Oklahoma State University, Tulsa,OK
Riley, Kate D.O, Department of Internal Medicine, Oklahoma State University, Tulsa, OK
Harris, Andrew D.O., Department of Internal Medicine, Oklahoma State University, Tulsa, OK
Corresponding Author:
Andrew Harris, D.O.
Email: drewharris1993@gmail.com
Address: 108 E Ithica Pl, Broken Arrow OK, 74012
Acknowledgements:
Author contributions:All authors contributed equally to this manuscript. All identifying information has been removed from this case report to protect patient privacy.
Conflicts of Interest: None to report
Financial disclosure/funding:
None to report
Introduction:
Schwannomas are typically benign tumors arising from schwann cells of cranial nerves, spinal nerve roots, or peripheral nerves. Schwann cells, which produce myelin, are a major cell type in the peripheral nervous system, performing critical roles in axonal maintenance, function, and regeneration of peripheral nerves.1 Although schwannomas have been reported in a multitude of locations including the head, neck, extremities, mediastinum, and retroperitoneum, pancreatic schwannomas (PS) are rare with only around 50 case reports.2 In 2004, Paranjape et. al., presented a review of 39 PS cases and found an average age of 57.75 years with a median size 8.79 cm and most commonly (40%) located in the pancreatic head. They also found that 36.7% were described as solid and 47.6% were described as cystic.3 Presenting symptoms of patients with PS include abdominal pain, nausea, vomiting, weight loss, and jaundice. Most PS are slow growing and encapsulated, with well defined margins. In cytological exam, schwannomas are spindle shaped cells with vague cytoplasmic boundaries embedded in a fibrillar wavy nucleus. S100 immunochemical stain is seen in PS, although not specific to schwannomas.4 The differential for PS includes pancreatic cystic neoplasms, neuroendocrine tumors, pancreatic pseudocyst, intraductal papillary mucinous neoplasm, cystadenocarcinoma, and lymphoma. Pancreatic schwannomas can be incidental on computed tomography (CT) but require tissue for diagnosis via endoscopic ultrasound (EUS), guided fine needle aspiration (FNA), or biopsy. Although the risk for malignant PS is small, benign PS can cause complications due to their size, thus surgical resection is recommended. Surgical approaches vary and range from enucleation to pancreaticojejunostomy.
Case Report:
This is a unique case of a 56-year-old man with a pancreatic schwannoma with a history of Diffuse Large B-cell Lymphoma (DLBCL), Follicular Low-Grade Lymphoma, and Barrett’s Esophagus. In 2009, the patient was diagnosed with stage 3-S DLBCL, involving intra-abdominal and retroperitoneal nodes with splenic involvement. He was treated with 6 courses of Rituximab, Cyclophosphamide, Doxorubicin Hydrochloride, Vincristine Sulfate, and Prednisone (R-CHOP) therapy with complete response. In September 2015, he underwent liver ultrasound which revealed no intrahepatic or extrahepatic duct abnormality. Three months later, the patient underwent a positron emission tomography scan (PET), that was consistent with adenopathy of a retroperitoneal lymph node, necessetating a biopsy of the lymph node in question as well as a liver biopsy. Biopsy of the liver showed minimal macrovesicular steatosis (less than 5%) without significant portal or lobular inflammation or significant fibrosis. Retroperitoneal lymph node biopsy showed low grade (grade 1-2) Follicular Lymphoma. Subsequent bone marrow biopsy flow cytometry was performed and was normal. In 2016, he was found to have progressive hypermetabolic adenopathy in the abdomen with a new lymph node noted in the left supraclavicular area, and the pt subsequently underwent treatment with Rituxan and Bendamustine. Patient also underwent maintenance therapy with Rituxan, which was administered every 3 months for a total of two years from 2017 to 2019 with no abnormal radiotracer accumulation and resolution of mesenteric uptake previously observed on PET scan. In 2018, the patient had persistent diarrhea and was diagnosed with Enteroaggregative E. Coli, Cyclospora Cayetanensis, and was treated with trimethoprim-sulfamethoxazole. He then underwent a screening colonoscopy and was found to have one 5 mm tubular adenoma in the proximal ascending colon. In March 2022, the patient underwent routine surveillance with a CT
scan which showed a new 1.1 cm hypodensity within the inferior aspect of the pancreas and haziness in the mesentery consistent with treated lymphoma. A few scattered mesenteric lymph nodes were detected without retroperitoneal adenopathy.
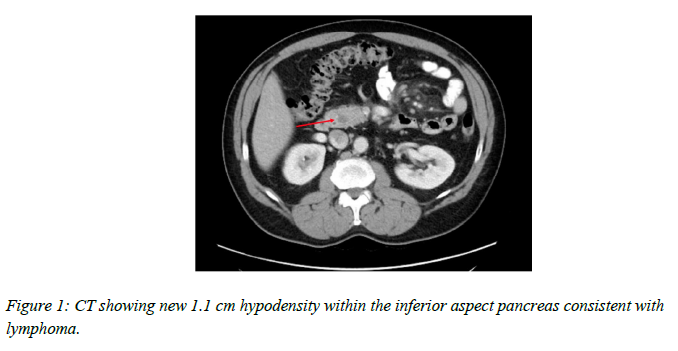
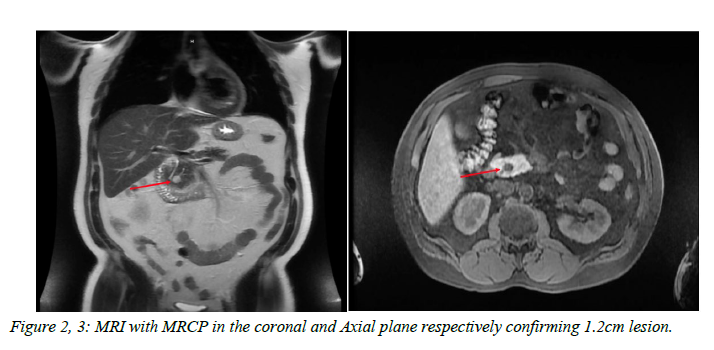
Magnetic resonance imaging (MRI) with magnetic resonance cholangiopancreatography (MRCP) was performed and confirmed an approximately 1.2 cm mass which showed delayed enhancement after intravenous contrast administration. There was no pancreatic or biliary ductal dilatation.
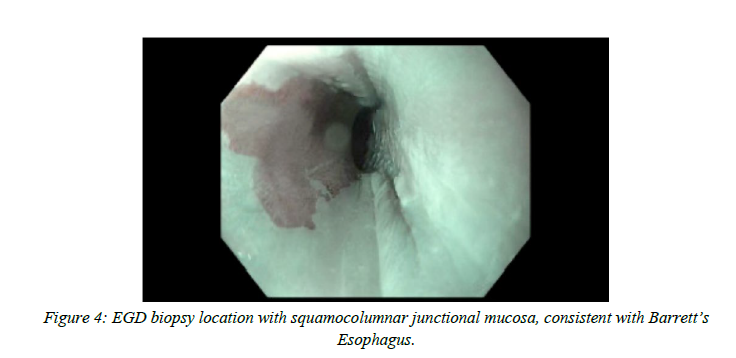
The patient was evaluated by gastroenterology and reported intermittent bloating. It was recommended that he undergo EUS with FNA and biopsy. Distal esophageal biopsy showed squamocolumnar junctional mucosa with inflammation and hyperplasia, consistent with reflux
esophagitis and was negative for intestinal metaplasia and dysplasia. Gastroesophageal junction biopsy showed squamocolumnar junctional mucosa with focal intestinal metaplasia, consistent with Barrett's Esophagus but negative for dysplasia and malignancy.
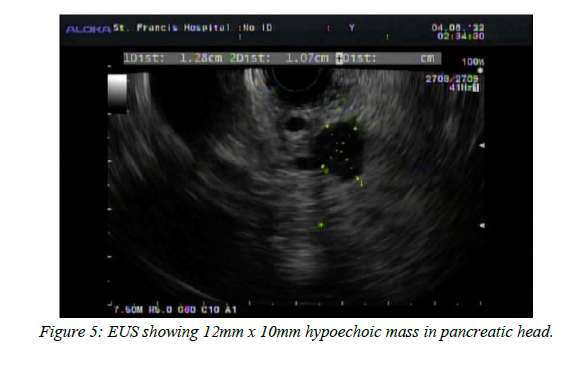
EUS of the pancreas showed a round hypoechoic mass identified in the pancreatic head measuring 12 mm x 10 mm in maximal cross-sectional diameter with well-defined borders. An intact interface was seen between the mass and the superior mesenteric artery, celiac trunk, and portal vein suggesting a lack of invasion
EUS-guided biopsy showed multiple fragments of bland lightly eosinophilic spindled cells present with very minimal pancreatic parenchyma in the background. Flow cytometry was performed and tested for CD5, CD10, CD19, CD20, CD45, Kappa, and Lambda. Immunohistochemical staining was performed and found to be positive for S-100 and CD34. Staining was negative for CD117(CKit), DOG-1, SMA, and CKAE1/3. FNA cytology showed glandular epithelial cells present with a spindle neoplasm. Given the patient’s clinical history, additional evaluation was requested at a specialized cancer center prior to consideration of surgical evaluation for possible resection which again confirmed the initial diagnosis of a pancreatic schwannoma. The patient is currently undergoing evaluation by otolaryngology for tinnitus and bilateral, left greater than right, sloping of mid to high frequency sensorineural hearing loss noted on audiometric evaluation.
Discussion:
Pancreatic schwannomas are rare and not extensively documented. There are case reports of B-cell Lymphoma mimicking schwannomas, however there does not appear to be a correlation between B-cell Lymphoma, Follicular Lymphoma, and incidence of peripheral schwannomas. Ayse et. al., reported a case of a breast schwannoma in a patient with DLBCL, however, to their knowledge, this was the first documented case of schwannoma co-existing with DLBCL.5 Pancreatic schwannomas may be misdiagnosed as pancreatic cyst lesions. They rarely degenerate and become malignant peripheral nerve sheath tumors in patients with von Recklinghausen disease. Extensive radical resection as opposed to simple enucleation for benign pancreatic schwannomas is recommended.6 In closing, while rare, pancreatic schwannoma is a diagnosis that merits consideration in patients with pancreatic lesions.
References:
1. Bhatheja, K., & Field, J. (2006). Schwann cells: Origins and role in Axonal Maintenance and regeneration. The International Journal of Biochemistry & Cell Biology, 38(12), 1995–1999. https://doi.org/10.1016/j.biocel.2006. 05.007
2. Ercan M, Aziret M, Bal A, et al. Pancreatic schwannoma: A rare case and a brief literature review. Int J Surg Case Rep. 2016;22:101-104. doi:10.1016/j.ijscr.2016.03.014
3. PARANJAPE, C., JOHNSON, S., KHWAJA, K., GOLDMAN, H., KRUSKAL, J., & HANTO, D. (2004). Clinical characteristics, treatment, and outcome of pancreatic schwannomas. Journal of Gastrointestinal Surgery, 8(6), 706–712. https://doi.org/10.1016/j.gassur.2004. 05.010
4. S100 - Pathology Dictionary. MyPathologyReport.ca. (n.d.). Retrieved April 26, 2022, from https://www.mypathologyreport.ca/s 100/
5. Salihoglu, A., Esatoglu, S. N., Eskazan, A. E., Halac, M., & Aydin, S. O. (2012). Breast Schwannoma in a patient with diffuse large B-cell lymphoma: A case report. Journal of Medical Case Reports, 6(1). https://doi.org/10.1186/1752-1947-6- 423
6. Ma Y, Shen B, Jia Y, et al. Pancreatic schwannoma: a case report and an updated 40-year review of the literature yielding 68 cases. BMC Cancer. 2017;17(1):853. Published 2017Dec14. doi:10.1186/s12885-017-3856-6