Candida auris: A Comprehensive Analysis of Clinical Trials
Rachel Wilkins, B.S., Oklahoma State University College of Osteopathic Medicine at Cherokee Nation, Office of Medical Student Research, Tahlequah, Oklahoma
Sarah Wilkinson, B.S., Oklahoma State University College of Osteopathic Center for Health Sciences, Office of Medical Student Research, Tulsa, Oklahoma
Eugenio Hernandez, Pharm D., Oklahoma State University Medical Center, Department of Pharmacy, Tulsa, Oklahoma
Benjamin Greiner, D.O., M.P.H.,Saint Francis Health System, Warren Clinic Internal Medicine, Tulsa, Oklahoma
Micah Hartwell, Ph.D. ,Oklahoma State University College of Osteopathic Medicine at Cherokee Nation, Office of Medical Student Research, Tahlequah, Oklahoma
Oklahoma State University Center for Health Sciences, Department of Psychiatry and Behavioral Sciences, Tulsa, Oklahoma
Corresponding Author: Rachel Wilkins, Oklahoma State University College of Osteopathic Medicine at the Cherokee Nation. Address: 19500 E Ross St, Tahlequah, OK 74464, United States. Email: racwilk@okstate.edu Phone: (918) 361-9838
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest/Declarations: Dr. Hartwell receives research funding from the National Institute of Child Health and Human Development (U54HD113173), Human Resources Services Administration (U4AMC44250-01-02 and R41MC45951 PI: Hartwell), and the National Institute of Justice (2020-R2-CX-0014).
Key Words: Candida auris, Clinical Trial, antimicrobial resistance
Abstract
Background: Candida auris (C. auris) was isolated in 2009 and has continued to increase in prevalence. Most strains exhibit multidrug resistance to primary antifungal treatments, increasing the difficulty of treatment by healthcare professionals. The World Health Organization designated C. auris on the fungal priority pathogen list and urges further research of treatment regimens to enhance scientific understanding and improve patient outcomes. Our goal was to analyze pertinent clinical trials regarding treatment of C. auris infections.
Methods: We analyzed the National Library of Medicine’s Clinical Trials (Clinicaltrials.gov; CTG) with a secondary search through MEDLINE (PubMed.gov). The search string used for CTG included the terms ‘Candida auris’ or ‘Invasive candidiasis” with ‘C. auris’ in the “other” field. Our search of PubMed used the following search string: ((clinical trial) AND (phase)) AND (candida auris).
Results: Few clinical trials have been started regarding C. auris and even fewer have published results with effective treatment modalities. Our search of CTG resulted in 7 unique trials with an additional 1 found using PubMed. Of these trials, 5 were interventional studies that used one of the following medications: fosmanogepix, oral ibrexafungerp (SCY-078), rezafungin, caspofungin, fluconazole, and intranasal 10% povidone-iodine. Of these, 2 trials have published results with anti-fungal treatments- NCT04148287 and NCT03667690.
Conclusion: Publishing the results of clinical trials promptly, increasing the amount of therapeutic research, and continuing further education of healthcare professionals and patients are essential in the development of an effective and safe cure for C. auris infections.
Introduction:
Candida auris (C. auris), a budding yeast associated with nosocomial infections, was first isolated and characterized in 2009 on the external ear canal of a patient in Japan.1,2 Since its initial identification, C. auris has now been located and identified across 41 countries on each continent except for Antarctica.2 This presents an escalating threat with invasive strains increasing in prevalence that has a wide variety of presentations includings urinary tract infections, blood infections, megitits, and amore- ultimately causing a crude rate mortality of 34%.2–4 The urgency of C. auris’ public health threat is compounded by the fact that most strains exhibit multidrug resistance to primary antifungal treatments, causing a plethora of complications for healthcare professionals. Notably, Chow et al. discovered that 80% of isolated strains display resistance to antifungal treatments such as fluconazole, 23% to amphotericin B, and 7% to micafungin.5
Recognizing the critical implications for public health, the World Health Organization (WHO) has classified C. auris as the second-highest-ranking fungal pathogen and designated its priority as critical in the 2022 WHO fungal priority pathogens list.3 The ranking criteria encompass considerations such as antifungal resistance, number of deaths caused by the pathogen, available treatment options, and diagnostic tools. This strategic classification aims to highlight fungal pathogens that pose a serious health risk, necessitating further research to enhance scientific understanding and improve patient outcomes.3 In response to the pressing need for enhanced C. auris treatment options, our study aims to provide a comprehensive summary of recent clinical trials focused on C. auris antifungal therapy. To achieve our objective, we analyzed emerging medical interventions found within the National Library of Medicine’s Clinical Trials (Clinicaltrials.gov; CTG) with a secondary search through PubMed.gov.
Methods:
To address our objectives, we conducted an observational analysis of clinical trials from two publicly available databases—CTG, and PubMed. Each of these searches were conducted on February 29, 2024.
Clinical trials database. CTG is an investigator-reported research database created and managed by the National Library of Medicine and the National Institution of Health. CTG facilitates public access to current clinical trials to improve knowledge and identify areas of improvement, and recruit potential study participants.6 Each study uploaded into the database contains information regarding the disease or condition they are studying, the treatment intervention they are using, primary and secondary endpoints, participant guidelines for enrollment, and study design.7 The search string used for CTG included the terms ‘Candida auris’ or ‘Invasive candidiasis” with ‘C. auris’ in the “other” field with trials that had an initial start date on or after January 1, 2009. Trials of all statuses and phases were selected by marking the appropriate check box. The remaining fields were left unchanged.
PubMed. We conducted a second search of PubMed (MEDLINE) to find additional clinical trials that included C. auris but did not appear using our search criteria on CTG. Our search of
PubMed for clinical trials that included C. auris used the following search string: (((clinical trial) AND (phase)) AND (candida auris).
Inclusion criteria, screening, and data extraction
Trials from the search were not included in our data if they did not specifically list Candida auris in the description, design, outcome measures, eligibility criteria, terms related to study, or medical subject heading (MeSH)terms. Articles must have had an associated National Clinical Trial (NCT) identification number– a unique 8-number code assigned to each trial registered on CTG8 – and have information connecting C. auris to the clinical trial to be included in our analysis. After conducting both searches, the results returned were first screened for inclusion criteria by two authors (R.W. and S.W.) in a masked duplicative fashion. Disagreements were resolved between the two authors with a third author (M.H.) acting as an arbiter. Data for the following characteristics were then extracted from the trials in the same manner by the same authors.
Data Analysis
From the initial searches, we reported the number of trials returned, the number excluded, and our final sample. We then reported the total number of participants included in the trials, whether they were observational or interventional, the potential mechanism for treatment, and the phase of the trials. For trials with results reported on CTG, we provide a summary of the latest results reported.
Results:
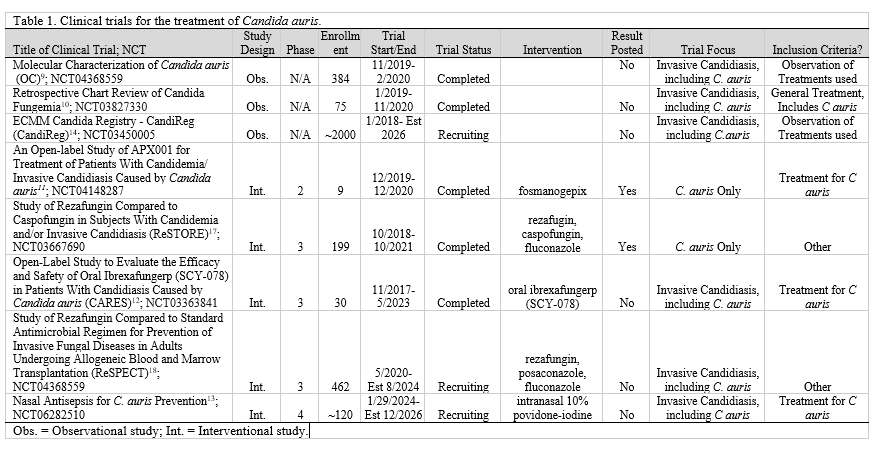
Our initial query of CTG for ‘C. auris’ resulted in 5 unique trials. 9–13 Our second CTG search for ‘invasive candidiasis’ resulted in 3 trials– 2 of which were excluded due to duplication from the initial search.11-12,14 Our search of PubMed returned 2 articles15-16 with 3 associated clinical trials— 1 was duplicated from the previous CTG search and was excluded.11,17-18 The other study was identified in CTG and added to our list, thus we examined 8 clinical trials for the treatment of C. auris (Table 1).9-13,17-18
Within the included studies, there were a total of 3279 enrolled participants—1159 actual participants from trials and 2120 estimated participants from Trial NCT03450005 and Trial NCT06282510, which were still in the recruiting phase. 9-13,17-18
By study design, three trials were listed as observational and five were interventional. The interventional studies included trial NCT06282510, trial NCT04148287, trial NCT03363841, trial NCT03667690, and trial NCT04368559. These trials used the following medications: intranasal 10% povidone iodine, fosmanogepix, oral ibrexafungerp (SCY-078), and rezafungin to test the efficacy against specific pathogens including C. auris.11-13,17-18 Trial NCT04121871 is an observational study where researchers are taking swabs from infected patients to test drug susceptibility using antifungal drug discs.9 Trial NCT03827330 is a retrospective observational study where researchers looked at patients who had a positive blood culture (Candida parapsilosis, Candida auris, Candida lusitaniae, Candida glabrata, and/or Candida albicans) at the National Institute of Health from 2004 to 2017 and what antifungal medications they were treated for the duration of infection.10 The final observation trial, NCT03450005, is a
prospective study making a database regarding drug susceptibility and treatment efficacy for Candida spp. infections.14
We found that out of the eight included trials, 6 had no results posted. Of these trials, three had the trial status “recruiting” and three were “completed.”9-10,12-14,18 The completed trials were finished in February 2020, November 2020, and May 2023.9-10,12 CTG results are not posted until they are submitted by the researcher—this could be due to project incompletion, the project’s deadline has yet to arrive, the submission is under view, or the researcher was not required to submit results to CTG.19-20
The two trials with results available on CTG were NCT04148287 and NCT03667690. Trial NCT04148287, investigating only C. auris with the intervention of fosmanogepix had 9 participants. Blood cultures were followed by 3 participants with a median of 3 days until the first negative cultures. By 2 weeks, 6 (66.7%) participants’ treatments were considered successful. By the end of the 6-week treatment period, 8 (88.9%) participants’ treatments were successful—meaning that no other antifungal therapy was used, completed two consecutive negative blood cultures, and one negative tissue culture and/or aspirate/fluid culture if applicable. By the end of the trial period and follow-up, all participants had experienced at least one adverse event that was not present before treatment had started, including all-cause mortality for 2 participants.11
Trial NCT03667690 compared rezafungin to caspofungin (and step-down oral fluconazole if applicable) in multiple invasive candidiasis species, including C. auris. There were 199 participants included in this trial, though only 93 in the rezafungin and 94 in the caspofungin groups were used for analyses reported in CTG (n=187). Global response data from appointments was assessed by the European Medicines Agency and the United States Food and Drug Administration. By the end of treatment (~day 30, less than 2 days from last dose received), 56 (60.2%) participants taking rezafungin were cured compared to 59 (62.8%) taking caspofungin. At follow-up (days 52-59), the number of participants cured was 42 (45.2%) and 39 (41.5%) respectively; criteria included for this is described as “an efficacious outcome and the desired results” by both data review committees. During the trials, 20 (21.5%) participants in the rezafungin and 17 (18.1%) participants in the caspofungin group had at least one treatment-emergent adverse event. All-cause mortality was found in 29 (31.2%) participants treated with rezafungin and 25 (26.6%) with caspofungin.17
Discussion:
Our results showed few clinical trials have been started regarding C. auris and even fewer have published results with effective treatment modalities. Our search of CTG resulted in 7 unique trials with an additional 1 found using PubMed. Of these trials, 3 were retrospective observational studies on interventions used previously for individuals infected by C. auris and 5 were interventional studies that used one of the following medications: fosmanogepix, oral ibrexafungerp (SCY-078), rezafungin, caspofungin, fluconazole, and intranasal 10% povidone-iodine.9-13,17-18 Trials NCT04368559, NCT03827330, NCT04148287, NCT03363841, and NCT03667690 have a posted status of completed.9-12,17 Trials NCT04148287 and NCT03667690 have results with anti-fungal treatment posted on CTG.11,17
Only two of the studies had results reported—the first showing that 8 of 9 patients treated with trial drug APX001 reported favorable outcomes, while the other study showed that 45.2% treated with rezafungin and 41.5% treated with caspofungin had favorable results.11,17 The latter study also showed a high prevalence of adverse events and mortality within both groups. While novel interventions may be on the horizon, such as APX001, the current first-line approaches recommended by the CDC include the use of echinocandins.21 These echinocandins include anidulafungin, caspofungin, or micafungin in adults—the latter two of which are also recommended in pediatric cases.21 When echinocandins do not provide favorable results, liposomal amphotericin B may be considered.21 As with the management of other Candida species, other medical treatment options should follow the Clinical Practice Guideline for the Management of Candidiasis.22
The implications of this study suggest further efforts must be taken to halt the progression of multidrug-resistant C. auris. These efforts should be multifaceted and include components of
physician education, facility-driven antimicrobial stewardship, and policy changes. The selection for multidrug-resistant organisms results from improper or excessive antimicrobial use in rapidly growing populations. Therefore, appropriate education in clinicians on antimicrobial stewardship and the growth of multidrug-resistant organisms could reasonably reduce the burden of these infections. Guidance for initiating this education has been previously published.23 While the Joint Commission has mandated that all accredited hospitals must have antimicrobial stewardship programs, funding of these programs is frequently a barrier to compliance. Therefore, policy changes must occur at the state and national levels to improve funding and discourage indiscriminate use of antimicrobials in plant and animal agriculture, both of which have been identified as causes for the current era of antimicrobial resistance.24
The emergence of several MDR microorganisms presents a current and future challenge for medical professionals and patients. Medical professionals can proactively address and prevent the development of additional MDR organisms by adhering to appropriate treatment guidelines to ensure the proper antimicrobial is selected at the optimal dose and duration. Additionally, they can provide education to patients regarding the appropriate use of antimicrobials and which medical conditions require their use.25 They can participate in antimicrobial stewardship programs that offer collaboration among clinicians, nursing staff, pharmacists, infection prevention teams, microbiologists, and patient safety teams to prescribe the proper antimicrobials effectively. Antimicrobial stewardship programs analyze four different components before prescribing antimicrobials—1) are antimicrobials required, 2) what is the most effective empiric treatment, 3) does the patient display signs of improvement after initial treatment, and 4) the duration of an antimicrobial course.26 This collaboration is crucial as medical professionals are the frontline in safeguarding the effectiveness of antimicrobial medications.27 Patients must ensure they are prioritizing their health by practicing effective hand hygiene and receiving the recommended vaccinations. Both of these maneuvers limit the need for antimicrobials by preventing an initial infection. If antimicrobials are prescribed by a clinician, it is vital to complete the entire course even if symptoms improve. Additionally, patients must refrain from sharing or using leftover antibiotics from previous prescriptions.25
Limitations:
A limitation of this study is that it only included trials registered in CTG, whether found through CTG or PubMed, thus limiting our scope and ability to analyze all trials conducted surrounding C. auris. CTG does not require all clinical studies in the United States to be registered. Specific trials not included are observational clinical research such as cohort studies, phase 1 trials of investigational drug products, small clinical trials used to determine the feasibility of a prototype device, and trials that focus on behavioral interventions. Trial updates are also limited. ClinicalTrials.gov allows studies to upload information either throughout the study or after the study is completed, thus preventing access to the information in a timely manner. These limitations have been mitigated by the increasing number of study registrations, coupled with the implementation of additional policies mandating the registration and implementing guidelines of these studies.
Conclusion:
Invasive strains of C. auris have increased in prevalence, causing a 34% mortality rate and an ongoing threat for healthcare professionals. As C. auris has developed resistance to multiple commonly used antifungal therapies, clinical trials are necessary to test novel treatments. Our search for clinical trials for C. auris resulted in 8 trials, for which only 2 trials reported results. The first study showed that 8 of 9 participants treated with APX001 reported favorable outcomes, the other study showed that less than half of participants receiving rezafungin or caspofungin had favorable results. These results indicate that it may be better to use other first line echinocandins such as caspofungin and micafungin because of increased adverse effects and mortality. Publishing the results of clinical trials promptly, increasing the amount of therapeutic research, and continuing further education of healthcare professionals and patients are essential in the development of an effective and safe cure for C. auris infections.
References
1. Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol. 2009;53(1):41-44. doi:10.1111/j.1348-0421.2008.00083.x
2. Sikora A, Hashmi MF, Zahra F. Candida Auris. StatPearls Publishing; 2023. Accessed January 23, 2024. https://www.ncbi.nlm.nih.gov/books/NBK563297/
3. World Health Organization. Antimicrobial Resistance Division, World Health Organization. Control of Neglected Tropical Diseases, World Health Organization. Global Coordination and Partnership, Alastruey-Izquierdo A. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. Organización Mundial de la Salud (OMS); 2022. Accessed January 24, 2024. https://repisalud.isciii.es/handle/20.500.12105/15113
4. Benedict K, Forsberg K, Gold JAW, Baggs J, Lyman M. Candida auris‒Associated Hospitalizations, United States, 2017-2022. Emerg Infect Dis. 2023;29(7):1485-1487. doi:10.3201/eid2907.230540
5. Chow NA, Muñoz JF, Gade L, et al. Tracing the Evolutionary History and Global Expansion of Candida auris Using Population Genomic Analyses. MBio. 2020;11(2). doi:10.1128/mBio.03364-19
6. ClinicalTrials.Gov. Accessed January 24, 2024. https://clinicaltrials.gov/about-site/about-ctg
7. ClinicalTrials.gov Background. Accessed March 14, 2024. https://classic.clinicaltrials.gov/ct2/about-site/background
8. Glossary of common site terms. Accessed April 18, 2024. https://classic.clinicaltrials.gov/ct2/about-studies/glossary
9. ClinicalTrials.gov. Accessed February 29, 2024. https://clinicaltrials.gov/study/NCT04121871?cond=candida%20auris&rank=1
10. ClinicalTrials.gov. Accessed February 29, 2024. https://clinicaltrials.gov/study/NCT03827330?cond=candida%20auris&rank=2
11. ClinicalTrials.gov. Accessed February 29, 2024. https://clinicaltrials.gov/study/NCT04148287?cond=candida%20auris&rank=3
12. ClinicalTrials.gov. Accessed February 29, 2024. https://clinicaltrials.gov/study/NCT03363841?cond=candida%20auris&rank=4
13. ClinicalTrials.gov. Accessed February 29, 2024. https://clinicaltrials.gov/study/NCT06282510?cond=candida%20auris&rank=1
14. ClinicalTrials.gov. Accessed February 29, 2024. https://clinicaltrials.gov/study/NCT03450005?cond=Invasive%20Candidiasis&term=candida%20auris&rank=1
15. Vazquez JA, Pappas PG, Boffard K, et al. Clinical Efficacy and Safety of a Novel Antifungal, Fosmanogepix, in Patients with Candidemia Caused by Candida auris : Results from a Phase 2 Trial. Antimicrob Agents Chemother. 2023;67(5). doi:10.1128/aac.01419-22
16. Ham YY, Lewis JS, Thompson GR. Rezafungin: a novel antifungal for the treatment of invasive candidiasis. Future Microbiol. 2021;16(1). doi:10.2217/fmb-2020-0217
17. ClinicalTrials.gov. Accessed April 16, 2024. https://clinicaltrials.gov/study/NCT03667690
18. ClinicalTrials.gov. Accessed April 16, 2024. https://clinicaltrials.gov/study/NCT04368559
19. ClinicalTrials.gov. Accessed March 14, 2024. https://clinicaltrials.gov/policy/faq
20. ClinicalTrials.gov. Accessed March 14, 2024.
21. Treatment and Management of C. auris Infections and Colonization. Published April 5, 2023. Accessed May 1, 2024. https://www.cdc.gov/fungal/candida-auris/c-auris-treatment.html
22. Pappas PG, Kauffman CA, Andes DR, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1-e50. doi:10.1093/cid/civ933
23. Luther VP, Shnekendorf R, Abbo LM, et al. Antimicrobial Stewardship Training for Infectious Diseases Fellows: Program Directors Identify a Curriculum Need. Clin Infect Dis. 2018;67(8):1285. doi:10.1093/cid/ciy332
24. Miller SA, Ferreira JP, LeJeune JT. Antimicrobial Use and Resistance in Plant Agriculture: A One Health Perspective. Collect FAO Agric. 2022;12(2):289. doi:10.3390/agriculture12020289
25. Antibiotic Resistance - NFID. Published online November 7, 2023. Accessed April 11, 2024. https://www.nfid.org/antibiotic-resistance/
26. Shrestha J, Zahra F, Cannady P Jr. Antimicrobial Stewardship. In: StatPearls [Internet]. StatPearls Publishing; 2023. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK572068/
27. Antimicrobial resistance. Accessed April 11, 2024. https://www.who.int/news-room/fact- sheets/detail/antimicrobial-resistance