Metastatic Papillary Thyroid Cancer: A Rare Variant and Clinical Presentation
Cody Bahavar, MSIV), Oklahoma State University Center for Health Sciences, Tulsa, OK
Yoon Cho D.O., Radiology Resident, Oklahoma State University Center for Health Sciences, Tulsa, OK
Jeff Lee, D.O., Radiology Resident, Oklahoma State University Center for Health Sciences, Tulsa, OK
Donald von Borstel, D.O. Attending Radiologist, Oklahoma State University Center for Health
Abstract
The incidence of papillary thyroid cancer has been increasing at a rate faster than any other thyroid malignancy and accounts for the majority of all thyroid neoplasms (75-85%). It is also the most common type of thyroid cancer to develop from prior radiation exposure.1 Radiology plays a crucial role in the diagnosis of papillary thyroid cancer, with ultrasound being one of the initial steps in the diagnostic workup. In addition, radioiodine (I-131) nuclear medicine studies can be used to evaluate for distant metastases as well as a treatment modality in ablating thyroid cancer. Thyroid lobectomy is considered the mainstay for treatment, with total thyroidectomy being reserved for higher risk tumors. Our case study illustrates the atypical clinical presentation, diagnostic evaluation, and therapeutic intervention of a patient with metastatic diffuse sclerosing variant of papillary thyroid carcinoma, which is a rare and aggressive subtype.
Introduction
Papillary thyroid cancer typically occurs in caucasions and has a female predilection (3:1 ratio), with a peak age of incidence between 25-50 years-old. It commonly presents as a painless neck mass. While no specific laboratory tests exist for diagnosis, elevation in thyroxine, triiodothyronine, or thyroid stimulating hormone (TSH) can be seen. Diffuse sclerosing variant of papillary thyroid carcinoma (DSPC) is a rare and aggressive variant of papillary thyroid carcinoma that consists of about 0.7-6.6% of all papillary thyroid carcinoma.2 This variant is seen in patients between the ages of 19.5 - 34.7 years with a predilection for females.3 DSPC often presents with cervical lymph node metastases, which is a poor prognostic factor. Also, there are cases of patients with DSPC presenting with symptoms that mimic thyroiditis, which is vastly different from other papillary thyroid carcinoma clinical presentations. Another important distinction for DSPC from conventional papillary thyroid carcinoma is that DSPC lacks BRAF mutation; which occurs uniquely in papillary thyroid cancer. Instead, RET/PTC rearrangement is the most specific marker that is beneficial as it can be used for targeted therapy of DSPC.4
We report an unusual clinical presentation of the diffuse sclerosing variant of papillary thyroid cancer in a 16-year-old male, who presented with a painless neck mass that had been growing over the past year. The patient subsequently received a total thyroidectomy and radioiodine treatment.
Key Words: Papillary, Thyroid Cancer, Diffuse Sclerosing Variant
Case Report
A 16-year-old Caucasian male with a family history of Graves disease presented to the Ear Nose and Throat clinic for a painless neck mass that had been progressively growing over the past year. The patient was asymptomatic otherwise. Two lower neck masses were palpated on physical exam. A computed tomography (CT) of the neck with contrast was initially ordered (Figure 1). The left thyroid showed a centrally necrotic 5 x 2.6 centimeter mass. The patient also had extensive lymphadenopathy extending from above the hyoid bone to the retrosternocleidomastoid fascia plane of the neck.
Figure 1.
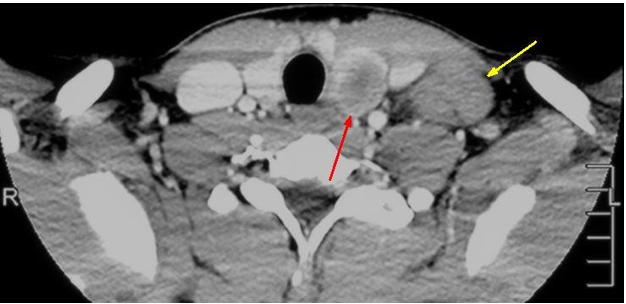
Figure 1. Axial post-contrast CT image at the level of the thyroid demonstrating a centrally necrotic left thyroid mass (red arrow displaying central hypodensity) with adjacent lobular lymphadenopathy (yellow arrow).
The combination of the thyroid mass and pathologic appearing lymphadenopathy was concerning for thyroid malignancy with metastatic disease, so ultrasound-guided fine needle aspiration (FNA) of the left thyroid was ordered (Figure 2), along with a biopsy of the enlarged left lymph node.
Figure 2.
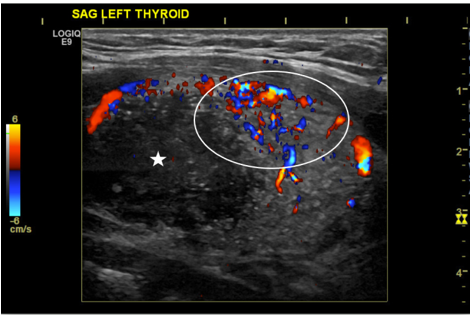
Figure 2. Pre-procedural grayscale and color doppler image of the left thyroid demonstrating a large hypervascular mass (white circle denoting hypervascularity) with central hypoechoic necrosis (white star).
Figure 3.
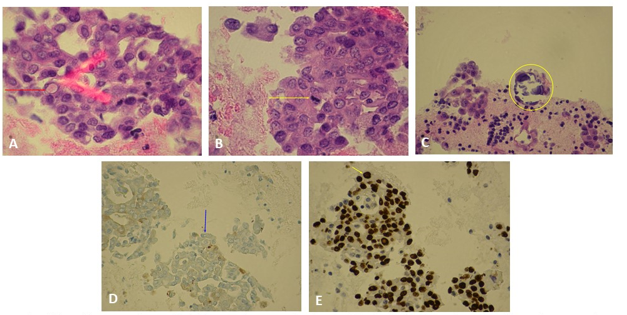
A.Thyroid FNA demonstrating a cell with intranuclear cytoplasmic pseudoinclusion (red arrow). B. Thyroid FNA demonstrating a cell with mitotic figures (yellow arrow). C. Thyroid FNA demonstrating a psammonstrating a psammonma body (yellow circle). D. Thyroid FNA thyroglobulin stain (blue)demonstrating thyroid tissue(blue arrow). E. Thyroid FNA TTF-1 stain demonstrating (brown) nuclear staining of cancer cells (yellow arrow).
Figure 3. Histology slides from thyroid fine needle aspiration, as annotated above.
The FNA revealed malignant cells identified in groups with irregular nuclei, nuclear grooves, and intranuclear cytoplasmic pseudoinclusions (Figure 3)- which is consistent with papillary thyroid cancer. The lymph node biopsy showed metastatic thyroid cancer and stained positive for thyroglobulin and thyroid transcription factor 1 (TTF1), both of which are seen in papillary thyroid carcinoma.
After the diagnosis was established, the decision was made to undergo a total thyroidectomy and excision of the affected lymph nodes. A large tumor was noted on the superior portion of the left lobe of the thyroid, which was surrounded by bulky lymph nodes (Figure 4). A total of 25 lymph nodes were examined by a pathologist,18 of which were found to be positive for papillary thyroid cancer markers. Immunohistological evaluation of the tumor was performed, which stained positive for keratin CK19, P63, TTF1, and high-molecular-weight keratin - all of which are markers for papillary thyroid cancer. The gross specimen of the thyroid mass revealed a rare and aggressive variant of papillary thyroid cancer, known as the diffuse sclerosing variant.
The patient tolerated the procedure well and was noted to have a strong cough and normal phonation afterward. He was started on synthroid for TSH suppression and scheduled for radioactive iodine (I-131) therapy. A nuclear medicine scan was performed two weeks after I-131 therapy and showed no evidence of residual metastasis.
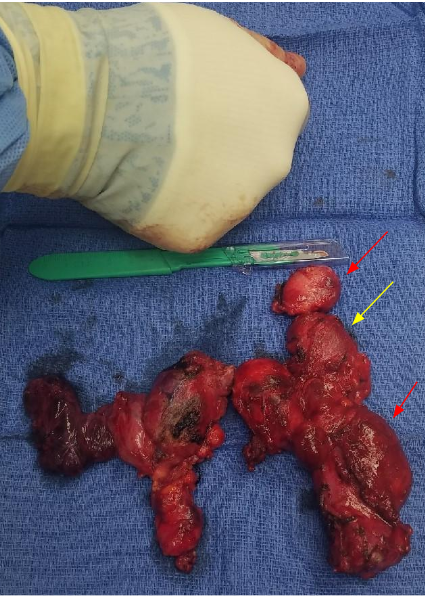
Figure 4. Gross specimen of the total thyroidectomy with central neck and left neck lymph nodes. Left thyroid lobe contains the tumor (yellow arrow) with multiple bulky surrounding lymph nodes (red arrows).
Discussion
Papillary thyroid cancer accounts for the majority of thyroid neoplasms and is more common in middle-aged caucasian females.5 Risk factors include radiation exposure during childhood, first-degree relatives with thyroid cancer, and thyroid cancer syndromes such as multiple endocrine neoplasia type II.6 It typically presents as a painless neck mass. Approximately 2-10 percent of patients will have metastases beyond the neck at the time of diagnosis, most commonly to the lungs and skeletal bones. Mutations in the mitogen-activated protein kinase pathway (MAPK) have been shown to play a critical role in the development of papillary thyroid cancer, especially the mutations of the BRAF gene.7,8
The histological findings of classical papillary thyroid cancer may show cells with hypodense chromatin, intranuclear cytoplasmic pseudoinclusions, nuclear grooves, and/or mitotic figures. Psammoma bodies are a characteristic finding in this disease and are present in up to 50 percent of cases.9 They are the scarred remnants of tumor papillae that have infarcted. In terms of immunohistochemistry, papillary thyroid cancer is positive for thyroglobulin and keratin. HMBE-1, RET, TTF1, and CK19 are four immunohistological tumor markers that have been shown to be useful in the diagnosis of papillary thyroid cancer.10
Ultrasound is the primary modality used for imaging thyroid disease. One of the initial steps in diagnosing papillary thyroid cancer is obtaining an ultrasound to confirm the presence of nodules, assess the sonographic features of this disease, and determine if there are any extra nodules present that could be metastatic lesions. Although thyroid carcinoma may have varied presentations on ultrasound and can be difficult to distinguish from benign processes, the presence of hypoechogenicity, irregular borders, antiparallel orientation, and internal microcalcifications should raise the clinical suspicion for papillary thyroid carcinoma.11 Microcalcifications in particular are associated with papillary carcinoma, as they are the imaging representation of psammoma bodies.
Our patient had the diffuse sclerosing variant of papillary thyroid carcinoma which has a tendency to grow more rapidly and metastasize to the cervical lymph nodes and lungs more frequently than other types of thyroid carcinoma. It has a predilection for young females. On ultrasound, the diffuse sclerosing variant shows similar characteristics as other types of papillary thyroid carcinoma, but tends to involve both thyroid lobes and shows more internal microcalcifications. With aggressive resection and radioactive treatment, the prognosis is the same as other variants of papillary thyroid carcinoma; with a 10 year survival rate of 98 percent.12
Computed tomography findings of thyroid carcinoma are also variable and have similar characteristics as the sonographic findings mentioned above, including findings of a low density mass with irregular margins and microcalcifications. Magnetic resonance imaging of thyroid carcinoma demonstrates heterogeneous signal and enhancement with possibility of both T1- and T2-shortening. Additionally, thyroid carcinoma demonstrates decreased signal on apparent diffusion coefficient (ADC). I-131 scintigraphy is used to detect remnant thyroid tissue or metastatic disease after thyroidectomy.
Most papillary thyroid cancers are slow growing and typically have a favorable prognosis. The mean 5-year survival rate can be nearly 100 percent in patients with localized disease and approximately 56 percent in cases of distant metastasis. The treatment for papillary thyroid cancer typically includes a thyroid lobectomy for mild cases and total thyroidectomy with resection of surrounding lymph node chains for higher risk cases. TSH and serum thyroglobulin are typically measured four to six weeks post lobectomy/thyroidectomy to monitor for persistent metastatic disease.
Our case was unique in that the patient was a 16 year-old male with no history of radiation exposure or family history of papillary thyroid cancer. He also had a rare and aggressive variant of papillary thyroid cancer known as the diffuse sclerosing variant, which accounts for less than four percent of papillary thyroid carcinoma cases and is usually seen in young female patients.12 He was found to have metastatic ipsilateral adenopathy and was treated with a total thyroidectomy with subsequent I-131 therapy. He had no post-surgical complications and I-131 showed no signs of distant metastases three months later.
References
1. Schneider AB, Sarne DH. Long-term risks for thyroid cancer and other neoplasms after exposure to radiation. Nat Clin Pract Endocrinol Metab. 2005;1(2):82-91.
2. Pillai S, Gopalan V, Smith RA, Lam AK-Y. Diffuse sclerosing variant of papillary thyroid carcinoma-an update of its clinicopathological features and molecular biology. Crit Rev Oncol Hematol. 2015;94(1):64-73.
3. Jung HK, Hong SW, Kim E-K, Yoon JH, Kwak JY. Diffuse Sclerosing Variant of Papillary Thyroid Carcinoma. Journal of Ultrasound in Medicine. 2013;32(2):347-354. doi:10.7863/jum.2013.32.2.347
4. Sheu S-Y, Schwertheim S, Worm K, Grabellus F, Schmid KW. Diffuse sclerosing variant of papillary thyroid carcinoma: lack of BRAF mutation but occurrence of RET/PTC rearrangements. Modern Pathology. 2007;20(7):779-787. doi:10.1038/modpathol.3800797
5. Cancer of the Thyroid - Cancer Stat Facts. SEER. http://seer.cancer.gov/statfacts/html/thyro.html. Accessed August 13, 2019.
6. Pal T, Vogl FD, Chappuis PO, et al. Increased risk for nonmedullary thyroid cancer in the first degree relatives of prevalent cases of nonmedullary thyroid cancer: a hospital-based study. J Clin Endocrinol Metab. 2001;86(11):5307-5312.
7. Giannini R, Ugolini C, Lupi C, et al. The heterogeneous distribution of BRAF mutation supports the independent clonal origin of distinct tumor foci in multifocal papillary thyroid carcinoma. J Clin Endocrinol Metab. 2007;92(9):3511-3516.
8. Fagin JA. How thyroid tumors start and why it matters: kinase mutants as targets for solid cancer pharmacotherapy. J Endocrinol. 2004;183(2):249-256.
9. Kumar V, Abbas AK, Fausto N, Aster JC. Robbins & Cotran Pathologic Basis of Disease E-Book. Elsevier Health Sciences; 2009.
10. Cerilli LA, Mills SE, Rumpel CA, Dudley TH, Moskaluk CA. Interpretation of RET immunostaining in follicular lesions of the thyroid. Am J Clin Pathol. 2002;118(2):186-193.
11. Tessler FN, Middleton WD, Grant EG, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. Journal of the American College of Radiology. 2017;14(5):587-595. doi:10.1016/j.jacr.2017.01.046
12. Kim HS, Han B-K, Shin JH, et al. Papillary thyroid carcinoma of a diffuse sclerosing variant: ultrasonographic monitoring from a normal thyroid gland to mass formation. Korean J Radiol. 2010;11(5):579-582.